Abstract
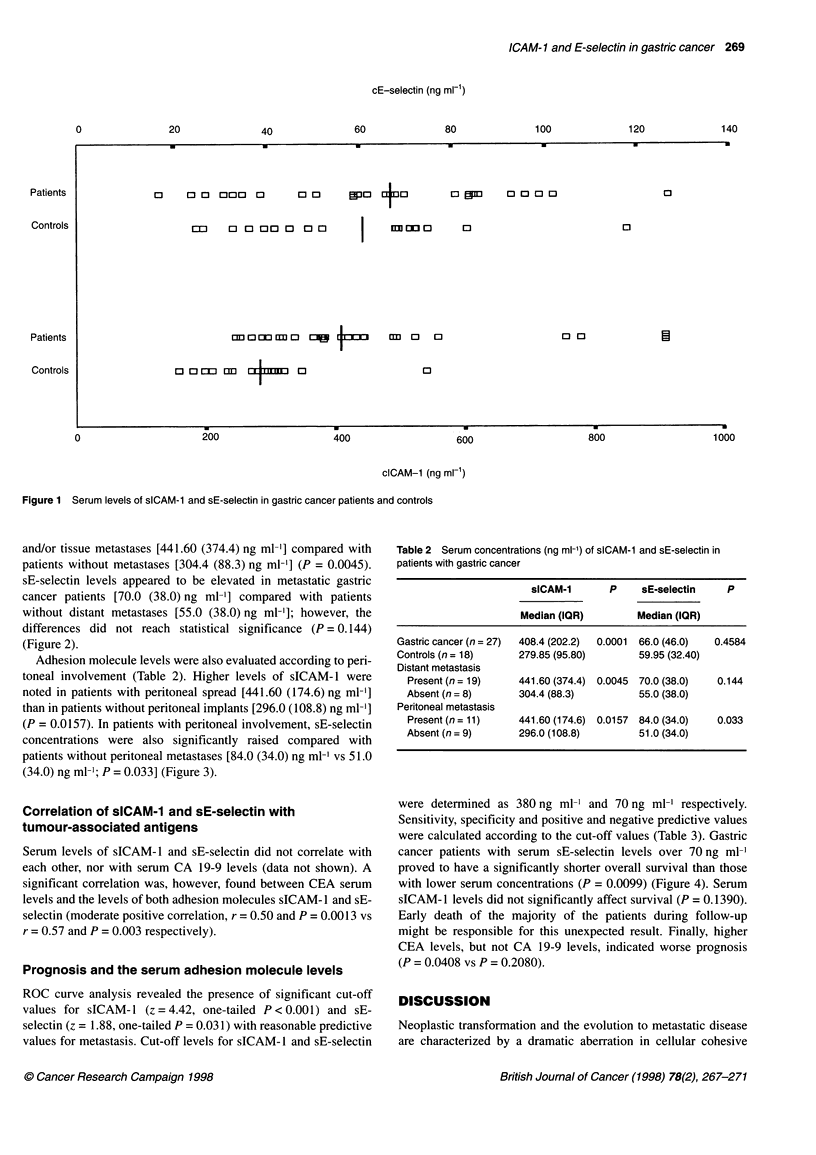
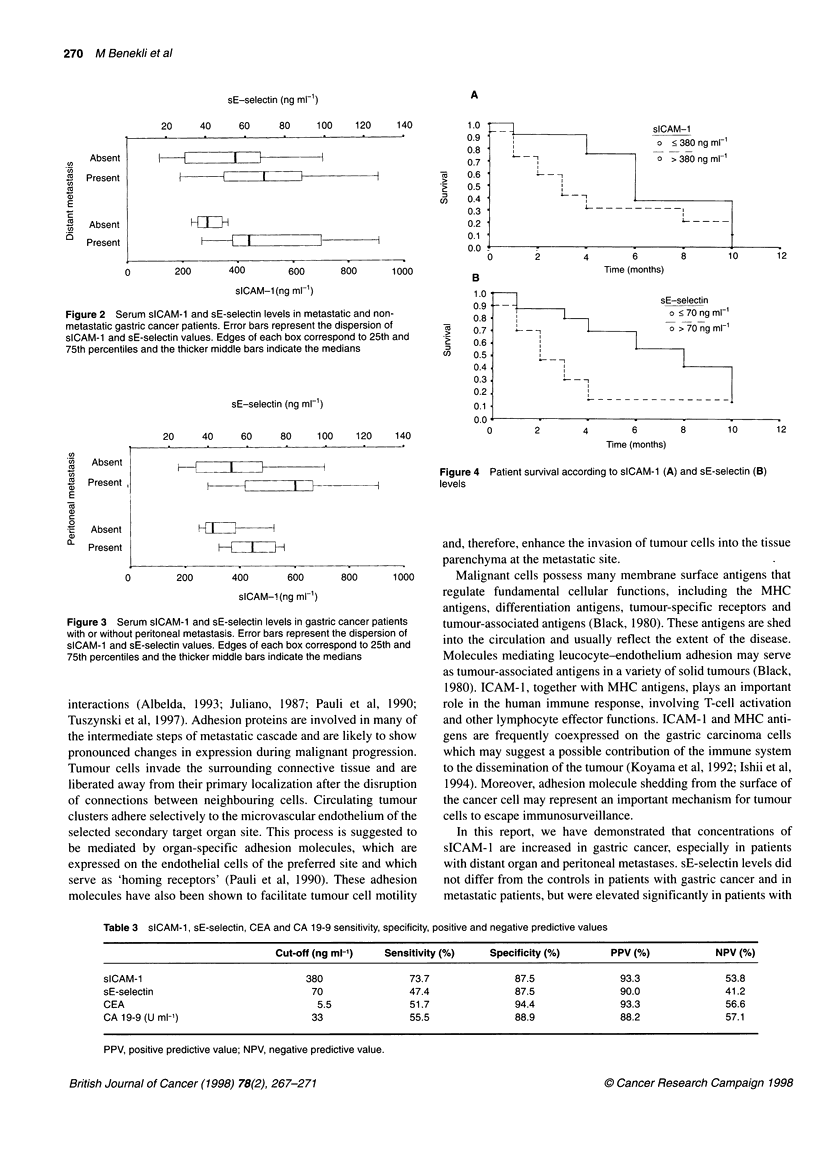
A diversity of adhesive interactions occur between the cancer cell and host extracellular matrix which potentiate neoplastic expansion and metastatic dissemination. In miscellaneous malignant diseases, tumour progression has been observed to be associated with alterations in adhesion molecule expression. Recently, circulating soluble intercellular adhesion molecules have been identified. In this study, serum levels of soluble intercellular adhesion molecule-1 (sICAM-1) and soluble E-selectin (sE-selectin) were determined in patients with gastric cancer. The study group consisted of 27 patients with previously untreated gastric adenocarcinoma. Four patients had stage II, two patients stage III and 21 patients stage IV disease according to the TNM classification. Nineteen patients had distant metastasis. The sera obtained from 18 healthy volunteers served as controls. Serum sICAM-1 and sE-selectin concentrations were determined by enzyme-linked immunosorbent assay (ELISA). In addition, we also studied other tumour-associated antigens, i.e. CEA and CA 19-9. Serum sICAM-1 levels were significantly increased in patients with gastric cancer (P < 0.0001). However, sE-selectin levels did not differ from the controls. sICAM-1 concentrations were also significantly higher in patients with distant metastasis and peritoneal spread (P = 0.0045 and P = 0.0157 respectively), whereas sE-Selectin levels were elevated only in patients with peritoneal metastasis (P = 0.033). Serum concentrations of sICAM-1 and sE-selectin correlated with CEA levels (P = 0.0013 and P = 0.003 respectively). Elevated levels of sE-selectin were associated with poorer prognosis (P = 0.0099), whereas sICAM-1 had no significant impact on survival. Our results suggest that increased sICAM-1 serum levels may reflect widespread disease and contribute directly to the progression of gastric cancer. Further investigation of the molecular mechanisms of adhesive tumour-host interactions may lead to a better understanding of the natural history of gastric cancer.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M. Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest. 1993 Jan;68(1):4–17. [PubMed] [Google Scholar]
- Banks R. E., Gearing A. J., Hemingway I. K., Norfolk D. R., Perren T. J., Selby P. J. Circulating intercellular adhesion molecule-1 (ICAM-1), E-selectin and vascular cell adhesion molecule-1 (VCAM-1) in human malignancies. Br J Cancer. 1993 Jul;68(1):122–124. doi: 10.1038/bjc.1993.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Nelson R. M. Selectins. J Clin Invest. 1993 Feb;91(2):379–387. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M., Butcher E., Furie B., Furie B., Gallatin M., Gimbrone M., Harlan J., Kishimoto K., Lasky L., McEver R. Selectins: a family of adhesion receptors. Cell. 1991 Oct 18;67(2):233–233. doi: 10.1016/0092-8674(91)90174-w. [DOI] [PubMed] [Google Scholar]
- Frenette P. S., Wagner D. D. Adhesion molecules--Part 1. N Engl J Med. 1996 Jun 6;334(23):1526–1529. doi: 10.1056/NEJM199606063342308. [DOI] [PubMed] [Google Scholar]
- Frenette P. S., Wagner D. D. Adhesion molecules--Part II: Blood vessels and blood cells. N Engl J Med. 1996 Jul 4;335(1):43–45. doi: 10.1056/NEJM199607043350108. [DOI] [PubMed] [Google Scholar]
- Honda M., Mori M., Ueo H., Sugimachi K., Akiyoshi T. Matrix metalloproteinase-7 expression in gastric carcinoma. Gut. 1996 Sep;39(3):444–448. doi: 10.1136/gut.39.3.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H., Gouchi A., Orita K. The enhancement of cell surface ICAM-I and HLA class I antigens in human gastric cancer cell lines by IFN-gamma. Acta Med Okayama. 1994 Apr;48(2):73–79. doi: 10.18926/AMO/31105. [DOI] [PubMed] [Google Scholar]
- Juliano R. L. Membrane receptors for extracellular matrix macromolecules: relationship to cell adhesion and tumor metastasis. Biochim Biophys Acta. 1987 Nov 25;907(3):261–278. doi: 10.1016/0304-419x(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Koyama S., Ebihara T., Fukao K. Expression of intercellular adhesion molecule 1 (ICAM-1) during the development of invasion and/or metastasis of gastric carcinoma. J Cancer Res Clin Oncol. 1992;118(8):609–614. doi: 10.1007/BF01211806. [DOI] [PubMed] [Google Scholar]
- Majuri M. L., Mattila P., Renkonen R. Recombinant E-selectin-protein mediates tumor cell adhesion via sialyl-Le(a) and sialyl-Le(x). Biochem Biophys Res Commun. 1992 Feb 14;182(3):1376–1382. doi: 10.1016/0006-291x(92)91885-t. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Mizoi T., Ohtani H., Suzuki Y., Shiiba K., Matsuno S., Nagura H. Intercellular adhesion molecule-1 expression by macrophages in human gastrointestinal carcinoma: possible roles as host immune/inflammatory reaction. Pathol Int. 1995 Aug;45(8):565–572. doi: 10.1111/j.1440-1827.1995.tb03504.x. [DOI] [PubMed] [Google Scholar]
- Nasu R., Mizuno M., Kiso T., Shimo K., Uesu T., Nasu J., Tomoda J., Okada H., Tsuji T. Immunohistochemical analysis of intercellular adhesion molecule-1 expression in human gastric adenoma and adenocarcinoma. Virchows Arch. 1997 Apr;430(4):279–283. doi: 10.1007/BF01092750. [DOI] [PubMed] [Google Scholar]
- Pauli B. U., Augustin-Voss H. G., el-Sabban M. E., Johnson R. C., Hammer D. A. Organ-preference of metastasis. The role of endothelial cell adhesion molecules. Cancer Metastasis Rev. 1990 Nov;9(3):175–189. doi: 10.1007/BF00046359. [DOI] [PubMed] [Google Scholar]
- Pui C. H., Luo X., Evans W., Martin S., Rugg A., Wilimas J., Crist W. M., Hudson M. Serum intercellular adhesion molecule-1 in childhood malignancy. Blood. 1993 Aug 1;82(3):895–898. [PubMed] [Google Scholar]
- Schwartz G. K. Invasion and metastases in gastric cancer: in vitro and in vivo models with clinical correlations. Semin Oncol. 1996 Jun;23(3):316–324. [PubMed] [Google Scholar]
- Tsujisaki M., Imai K., Hirata H., Hanzawa Y., Masuya J., Nakano T., Sugiyama T., Matsui M., Hinoda Y., Yachi A. Detection of circulating intercellular adhesion molecule-1 antigen in malignant diseases. Clin Exp Immunol. 1991 Jul;85(1):3–8. doi: 10.1111/j.1365-2249.1991.tb05673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski G. P., Wang T. N., Berger D. Adhesive proteins and the hematogenous spread of cancer. Acta Haematol. 1997;97(1-2):29–39. doi: 10.1159/000203657. [DOI] [PubMed] [Google Scholar]
- Ura H., Denno R., Hirata K. Correlation between nm23 protein and several cell adhesion molecules in human gastric carcinoma. Jpn J Cancer Res. 1996 May;87(5):512–517. doi: 10.1111/j.1349-7006.1996.tb00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasoshima T., Denno R., Kawaguchi S., Sato N., Okada Y., Ura H., Kikuchi K., Hirata K. Establishment and characterization of human gastric carcinoma lines with high metastatic potential in the liver: changes in integrin expression associated with the ability to metastasize in the liver of nude mice. Jpn J Cancer Res. 1996 Feb;87(2):153–160. doi: 10.1111/j.1349-7006.1996.tb03153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


