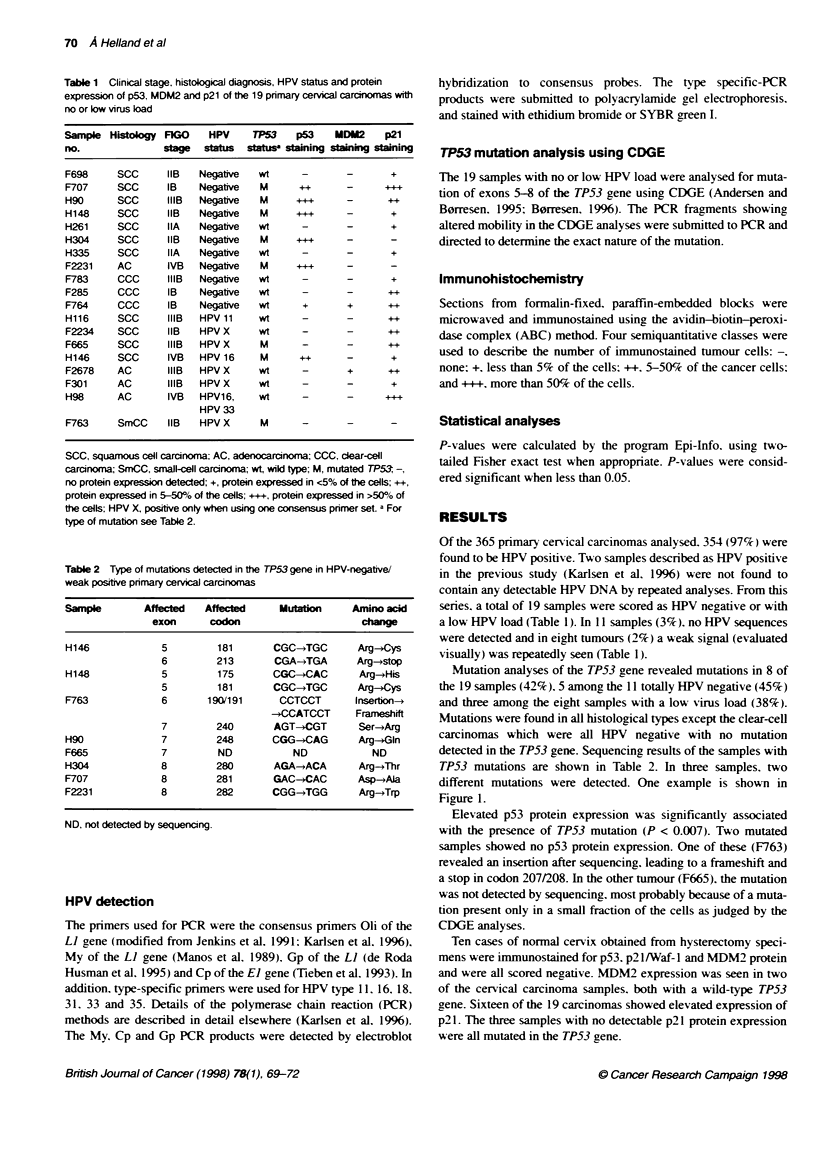
Abstract
Several studies have focused on the role of p53 inactivation in cervical cancer, either by inactivating mutations in the TP53 gene or by degradation of the p53 protein by human papillomavirus (HPV). In this study, primary cervical carcinomas from 365 patients were analysed for presence of HPV using both consensus primer-sets and type-specific primer-sets. Nineteen samples were determined to have no or low virus load, and were selected for further analyses: mutation screening of the TP53 gene using constant denaturant gel electrophoresis (CDGE) followed by sequencing, and protein expression of p53, MDM2 and p21 using immunohistochemistry (IHC). Mutations in the TP53 gene were found in eight samples (42%). Elevated p53 protein expression was significantly associated with presence of a mutation (P < 0.007). P21 protein expression was detected in 16 of the 19 carcinomas. No p21 expression was seen in normal cervical tissue. Two samples, both with wild-type p53, had elevated MDM2 expression. Compared with a previous study from our group, of mainly HPV-positive cervical carcinomas, in which only one sample was found to contain a TP53 mutation, a significantly higher mutation frequency (P < 0.001) was found among the carcinomas with no or low virus load. Although p53 inactivation pathways are not detected in every tumour, our study supports the hypothesis that p53 inactivation, either by binding to cellular or viral proteins or by mutation, is essential in the development of cervical carcinomas.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen T. I., Børresen A. L. Alterations of the TP53 gene as a potential prognostic marker in breast carcinomas. Advantages of using constant denaturant gel electrophoresis in mutation detection. Diagn Mol Pathol. 1995 Sep;4(3):203–211. doi: 10.1097/00019606-199509000-00008. [DOI] [PubMed] [Google Scholar]
- Bosch F. X., Manos M. M., Muñoz N., Sherman M., Jansen A. M., Peto J., Schiffman M. H., Moreno V., Kurman R., Shah K. V. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995 Jun 7;87(11):796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- Busby-Earle R. M., Steel C. M., Williams A. R., Cohen B., Bird C. C. p53 mutations in cervical carcinogenesis--low frequency and lack of correlation with human papillomavirus status. Br J Cancer. 1994 Apr;69(4):732–737. doi: 10.1038/bjc.1994.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Børresen A. L., Helland A., Nesland J., Holm R., Trope C., Kaern J. Papillomaviruses, p53, and cervical cancer. Lancet. 1992 May 30;339(8805):1350–1351. [PubMed] [Google Scholar]
- Chen T. M., Chen C. A., Hsieh C. Y., Chang D. Y., Chen Y. H., Defendi V. The state of p53 in primary human cervical carcinomas and its effects in human papillomavirus-immortalized human cervical cells. Oncogene. 1993 Jun;8(6):1511–1518. [PubMed] [Google Scholar]
- Choo K. B., Chong K. Y. Absence of mutation in the p53 and the retinoblastoma susceptibility genes in primary cervical carcinomas. Virology. 1993 Apr;193(2):1042–1046. doi: 10.1006/viro.1993.1224. [DOI] [PubMed] [Google Scholar]
- Crook T., Wrede D., Vousden K. H. p53 point mutation in HPV negative human cervical carcinoma cell lines. Oncogene. 1991 May;6(5):873–875. [PubMed] [Google Scholar]
- Dürst M., Glitz D., Schneider A., zur Hausen H. Human papillomavirus type 16 (HPV 16) gene expression and DNA replication in cervical neoplasia: analysis by in situ hybridization. Virology. 1992 Jul;189(1):132–140. doi: 10.1016/0042-6822(92)90688-l. [DOI] [PubMed] [Google Scholar]
- Fujita M., Inoue M., Tanizawa O., Iwamoto S., Enomoto T. Alterations of the p53 gene in human primary cervical carcinoma with and without human papillomavirus infection. Cancer Res. 1992 Oct 1;52(19):5323–5328. [PubMed] [Google Scholar]
- Helland A., Holm R., Kristensen G., Kaern J., Karlsen F., Trope C., Nesland J. M., Børresen A. L. Genetic alterations of the TP53 gene, p53 protein expression and HPV infection in primary cervical carcinomas. J Pathol. 1993 Oct;171(2):105–114. doi: 10.1002/path.1711710207. [DOI] [PubMed] [Google Scholar]
- Ikenberg H., Matthay K., Schmitt B., Bauknecht T., Kiechle-Schwarz M., Göppinger A., Pfleiderer A. p53 mutation and MDM2 amplification are rare even in human papillomavirus-negative cervical carcinomas. Cancer. 1995 Jul 1;76(1):57–66. doi: 10.1002/1097-0142(19950701)76:1<57::aid-cncr2820760108>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Iwasaka T., Oh-uchida M., Matsuo N., Yokoyama M., Fukuda K., Hara K., Fukuyama K., Hori K., Sugimori H. Correlation between HPV positivity and state of the p53 gene in cervical carcinoma cell lines. Gynecol Oncol. 1993 Jan;48(1):104–109. doi: 10.1006/gyno.1993.1016. [DOI] [PubMed] [Google Scholar]
- Jenkins A., Kristiansen B. E., Ask E., Oskarsen B., Kristiansen E., Lindqvist B., Trope C., Kjørstad K. Detection of genital papillomavirus types by polymerase chain reaction using common primers. APMIS. 1991 Jul;99(7):667–673. doi: 10.1111/j.1699-0463.1991.tb01244.x. [DOI] [PubMed] [Google Scholar]
- Jiko K., Tsuda H., Sato S., Hirohashi S. Pathogenetic significance of p53 and c-Ki-ras gene mutations and human papillomavirus DNA integration in adenocarcinoma of the uterine cervix and uterine isthmus. Int J Cancer. 1994 Dec 1;59(5):601–606. doi: 10.1002/ijc.2910590505. [DOI] [PubMed] [Google Scholar]
- Karlsen F., Kalantari M., Jenkins A., Pettersen E., Kristensen G., Holm R., Johansson B., Hagmar B. Use of multiple PCR primer sets for optimal detection of human papillomavirus. J Clin Microbiol. 1996 Sep;34(9):2095–2100. doi: 10.1128/jcm.34.9.2095-2100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubbutat M. H., Jones S. N., Vousden K. H. Regulation of p53 stability by Mdm2. Nature. 1997 May 15;387(6630):299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- Milde-Langosch K., Albrecht K., Joram S., Schlechte H., Giessing M., Löning T. Presence and persistence of HPV infection and p53 mutation in cancer of the cervix uteri and the vulva. Int J Cancer. 1995 Nov 27;63(5):639–645. doi: 10.1002/ijc.2910630507. [DOI] [PubMed] [Google Scholar]
- Miwa K., Miyamoto S., Kato H., Imamura T., Nishida M., Yoshikawa Y., Nagata Y., Wake N. The role of p53 inactivation in human cervical cell carcinoma development. Br J Cancer. 1995 Feb;71(2):219–226. doi: 10.1038/bjc.1995.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette R. L., Lee Y. Y., Wilczynski S. P., Karmakar A., Kizaki M., Miller C. W., Koeffler H. P. Mutations of p53 and human papillomavirus infection in cervical carcinoma. Cancer. 1993 Aug 15;72(4):1272–1280. doi: 10.1002/1097-0142(19930815)72:4<1272::aid-cncr2820720420>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Münger K., Byrne J. C., Howley P. M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Sidransky D., Tokino T., Helzlsouer K., Zehnbauer B., Rausch G., Shelton B., Prestigiacomo L., Vogelstein B., Davidson N. Inherited p53 gene mutations in breast cancer. Cancer Res. 1992 May 15;52(10):2984–2986. [PubMed] [Google Scholar]
- Srivastava S., Tong Y. A., Devadas K., Zou Z. Q., Chen Y., Pirollo K. F., Chang E. H. The status of the p53 gene in human papilloma virus positive or negative cervical carcinoma cell lines. Carcinogenesis. 1992 Jul;13(7):1273–1275. doi: 10.1093/carcin/13.7.1273. [DOI] [PubMed] [Google Scholar]
- Tieben L. M., ter Schegget J., Minnaar R. P., Bouwes Bavinck J. N., Berkhout R. J., Vermeer B. J., Jebbink M. F., Smits H. L. Detection of cutaneous and genital HPV types in clinical samples by PCR using consensus primers. J Virol Methods. 1993 May;42(2-3):265–279. doi: 10.1016/0166-0934(93)90038-s. [DOI] [PubMed] [Google Scholar]
- Tsuda H., Hirohashi S. Frequent occurrence of p53 gene mutations in uterine cancers at advanced clinical stage and with aggressive histological phenotypes. Jpn J Cancer Res. 1992 Nov;83(11):1184–1191. doi: 10.1111/j.1349-7006.1992.tb02743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roda Husman A. M., Walboomers J. M., van den Brule A. J., Meijer C. J., Snijders P. J. The use of general primers GP5 and GP6 elongated at their 3' ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995 Apr;76(Pt 4):1057–1062. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]