Abstract
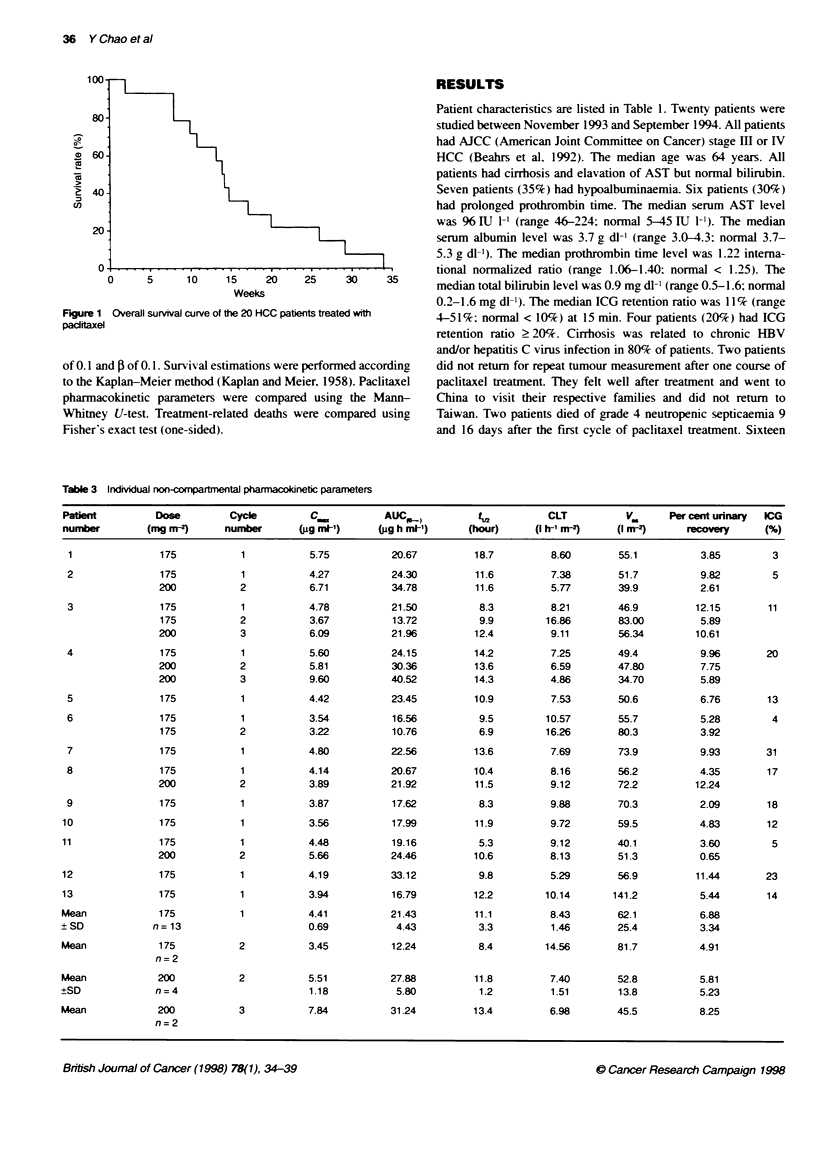
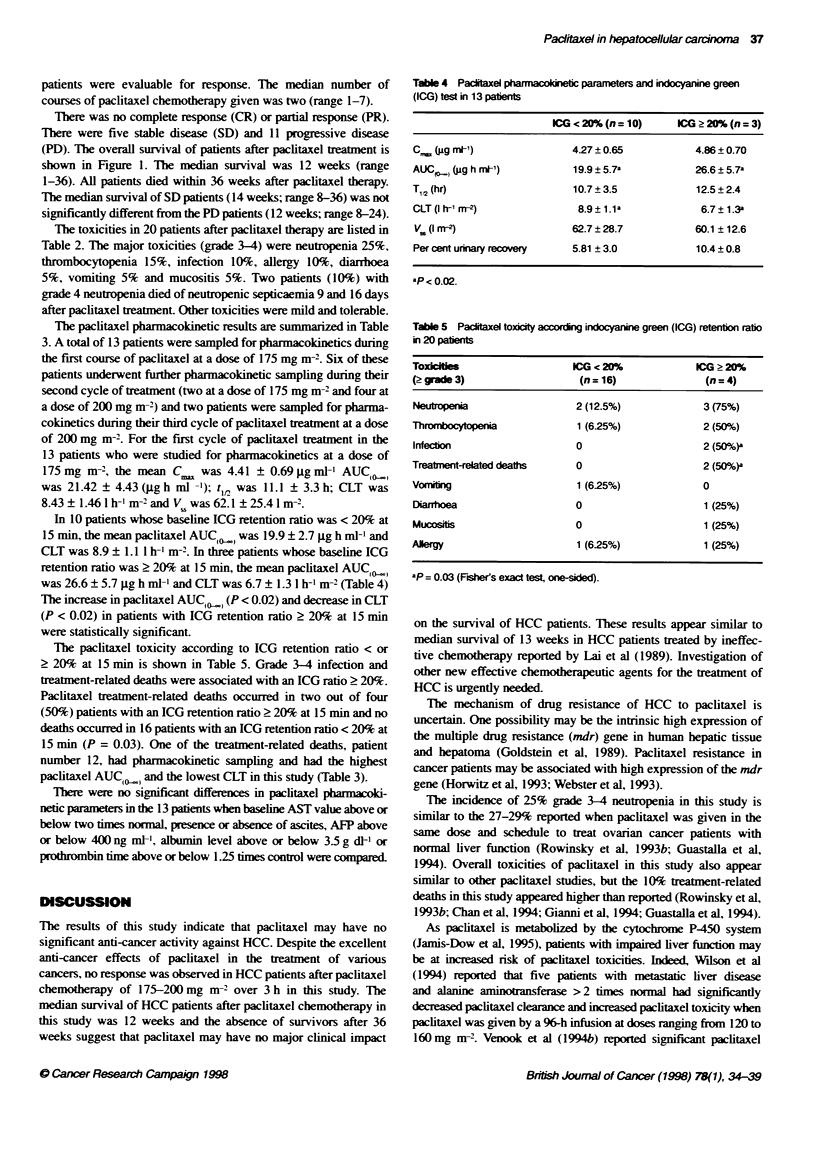
Hepatocellular carcinoma (HCC) is a common lethal disease in Asia and there is no effective chemotherapy. Identification of new effective drugs in the treatment of inoperable HCC is urgently need. This is a phase II clinical study to investigate the efficacy, toxicity and pharmacokinetics of paclitaxel in HCC patients. Twenty patients with measurable, unresectable HCC, normal serum bilirubin, normal bone marrow and renal functions were studied. Paclitaxel 175 mg m(-2) was given intravenously over 3 h every 3 weeks. No complete or partial responses were observed. Five patients had stable disease. Major treatment toxicities (grade 3-4) were neutropenia (25%), thrombocytopenia (15%), infection (10%) and allergy (10%). Treatment-related deaths occurred in two patients. The median survival was 12 weeks (range 1-36). Paclitaxel is metabolized by the liver and the pharmacokinetics of paclitaxel in cancer patients with liver involvement or impairment may be important clinically. Pharmacokinetic study was completed in 13 HCC patients. The paclitaxel area under the curve was significantly increased (P < 0.02), clearance decreased (P < 0.02) and treatment-related deaths increased (P = 0.03) in patients with hepatic impairment. In conclusion, paclitaxel in this dose and schedule has no significant anti-cancer effect in HCC patients. Paclitaxel should be used with caution in cancer patients with liver impairment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAESAR J., SHALDON S., CHIANDUSSI L., GUEVARA L., SHERLOCK S. The use of indocyanine green in the measurement of hepatic blood flow and as a test of hepatic function. Clin Sci. 1961 Aug;21:43–57. [PubMed] [Google Scholar]
- CHERRICK G. R., STEIN S. W., LEEVY C. M., DAVIDSON C. S. Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960 Apr;39:592–600. doi: 10.1172/JCI104072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni L., Capri G., Munzone E., Straneo M. Paclitaxel (Taxol) efficacy in patients with advanced breast cancer resistant to anthracyclines. Semin Oncol. 1994 Oct;21(5 Suppl 8):29–33. [PubMed] [Google Scholar]
- Goldstein L. J., Galski H., Fojo A., Willingham M., Lai S. L., Gazdar A., Pirker R., Green A., Crist W., Brodeur G. M. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst. 1989 Jan 18;81(2):116–124. doi: 10.1093/jnci/81.2.116. [DOI] [PubMed] [Google Scholar]
- Guchelaar H. J., ten Napel C. H., de Vries E. G., Mulder N. H. Clinical, toxicological and pharmaceutical aspects of the antineoplastic drug taxol: a review. Clin Oncol (R Coll Radiol) 1994;6(1):40–48. doi: 10.1016/s0936-6555(05)80367-x. [DOI] [PubMed] [Google Scholar]
- Horwitz S. B., Cohen D., Rao S., Ringel I., Shen H. J., Yang C. P. Taxol: mechanisms of action and resistance. J Natl Cancer Inst Monogr. 1993;(15):55–61. [PubMed] [Google Scholar]
- Jamis-Dow C. A., Klecker R. W., Katki A. G., Collins J. M. Metabolism of taxol by human and rat liver in vitro: a screen for drug interactions and interspecies differences. Cancer Chemother Pharmacol. 1995;36(2):107–114. doi: 10.1007/BF00689193. [DOI] [PubMed] [Google Scholar]
- Jwo S. C., Chiu J. H., Chau G. Y., Loong C. C., Lui W. Y. Risk factors linked to tumor recurrence of human hepatocellular carcinoma after hepatic resection. Hepatology. 1992 Dec;16(6):1367–1371. doi: 10.1002/hep.1840160611. [DOI] [PubMed] [Google Scholar]
- Lai K. H., Tsai Y. T., Lee S. D., Ng W. W., Teng H. C., Tam T. N., Lo G. H., Lin H. C., Lin H. J., Wu J. C. Phase II study of mitoxantrone in unresectable primary hepatocellular carcinoma following hepatitis B infection. Cancer Chemother Pharmacol. 1989;23(1):54–56. doi: 10.1007/BF00258459. [DOI] [PubMed] [Google Scholar]
- Okamoto E., Kyo A., Yamanaka N., Tanaka N., Kuwata K. Prediction of the safe limits of hepatectomy by combined volumetric and functional measurements in patients with impaired hepatic function. Surgery. 1984 May;95(5):586–592. [PubMed] [Google Scholar]
- Oken M. M., Creech R. H., Tormey D. C., Horton J., Davis T. E., McFadden E. T., Carbone P. P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982 Dec;5(6):649–655. [PubMed] [Google Scholar]
- Riegelman S., Collier P. The application of statistical moment theory to the evaluation of in vivo dissolution time and absorption time. J Pharmacokinet Biopharm. 1980 Oct;8(5):509–534. doi: 10.1007/BF01059549. [DOI] [PubMed] [Google Scholar]
- Rowinsky E. K., Eisenhauer E. A., Chaudhry V., Arbuck S. G., Donehower R. C. Clinical toxicities encountered with paclitaxel (Taxol). Semin Oncol. 1993 Aug;20(4 Suppl 3):1–15. [PubMed] [Google Scholar]
- Rowinsky E. K., Wright M., Monsarrat B., Lesser G. J., Donehower R. C. Taxol: pharmacology, metabolism and clinical implications. Cancer Surv. 1993;17:283–304. [PubMed] [Google Scholar]
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989 Mar;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Sonnichsen D. S., Hurwitz C. A., Pratt C. B., Shuster J. J., Relling M. V. Saturable pharmacokinetics and paclitaxel pharmacodynamics in children with solid tumors. J Clin Oncol. 1994 Mar;12(3):532–538. doi: 10.1200/JCO.1994.12.3.532. [DOI] [PubMed] [Google Scholar]
- Tong M. J., Sun S. C., Schaeffer B. T., Chang N. K., Lo K. J., Peters R. L. Hepatitis-associated antigen and hepatocellular carcinoma in Taiwan. Ann Intern Med. 1971 Nov;75(5):687–691. doi: 10.7326/0003-4819-75-5-687. [DOI] [PubMed] [Google Scholar]
- Venook A. P. Treatment of hepatocellular carcinoma: too many options? J Clin Oncol. 1994 Jun;12(6):1323–1334. doi: 10.1200/JCO.1994.12.6.1323. [DOI] [PubMed] [Google Scholar]
- Webster L., Linsenmeyer M., Millward M., Morton C., Bishop J., Woodcock D. Measurement of cremophor EL following taxol: plasma levels sufficient to reverse drug exclusion mediated by the multidrug-resistant phenotype. J Natl Cancer Inst. 1993 Oct 20;85(20):1685–1690. doi: 10.1093/jnci/85.20.1685. [DOI] [PubMed] [Google Scholar]
- Willey T. A., Bekos E. J., Gaver R. C., Duncan G. F., Tay L. K., Beijnen J. H., Farmen R. H. High-performance liquid chromatographic procedure for the quantitative determination of paclitaxel (Taxol) in human plasma. J Chromatogr. 1993 Nov 24;621(2):231–238. doi: 10.1016/0378-4347(93)80100-i. [DOI] [PubMed] [Google Scholar]
- Wilson W. H., Berg S. L., Bryant G., Wittes R. E., Bates S., Fojo A., Steinberg S. M., Goldspiel B. R., Herdt J., O'Shaughnessy J. Paclitaxel in doxorubicin-refractory or mitoxantrone-refractory breast cancer: a phase I/II trial of 96-hour infusion. J Clin Oncol. 1994 Aug;12(8):1621–1629. doi: 10.1200/JCO.1994.12.8.1621. [DOI] [PubMed] [Google Scholar]
- Wu C. C., Ho W. L., Yeh D. C., Huang C. R., Liu T. J., P'eng F. K. Hepatic resection of hepatocellular carcinoma in cirrhotic livers: is it unjustified in impaired liver function? Surgery. 1996 Jul;120(1):34–39. doi: 10.1016/s0039-6060(96)80238-8. [DOI] [PubMed] [Google Scholar]


