Abstract
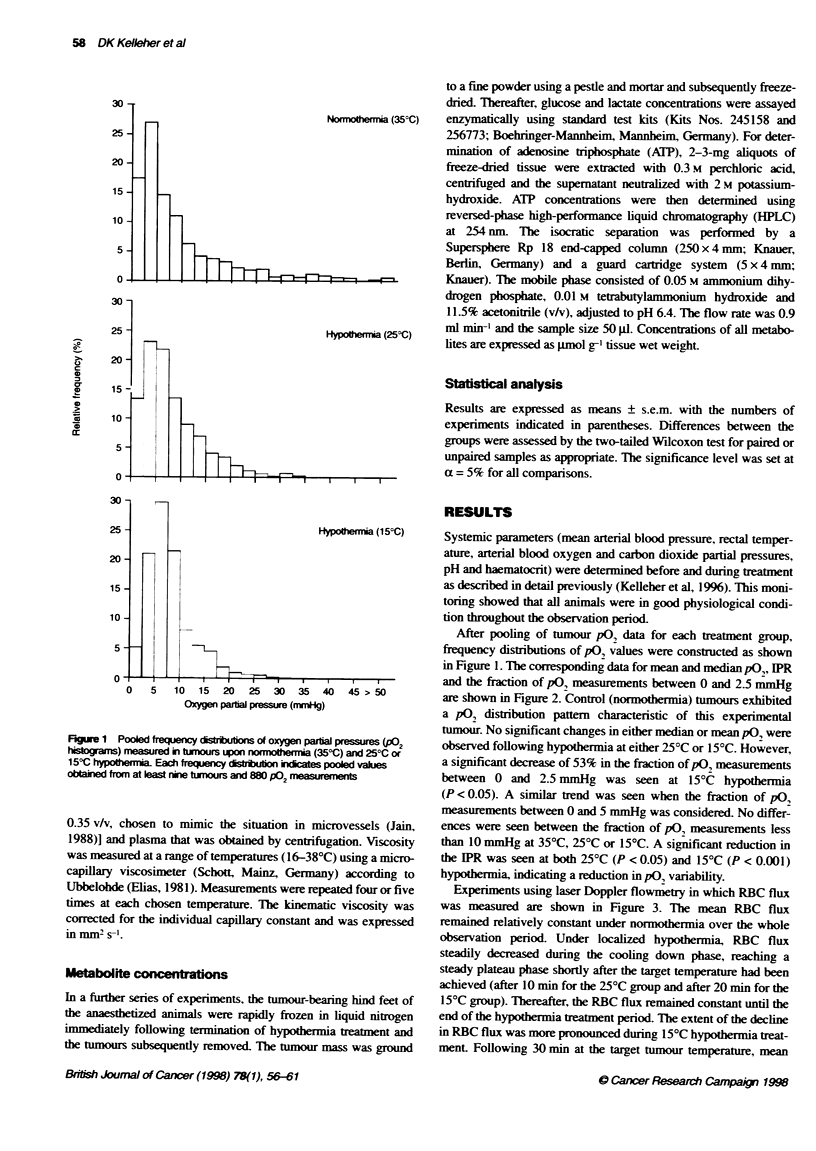
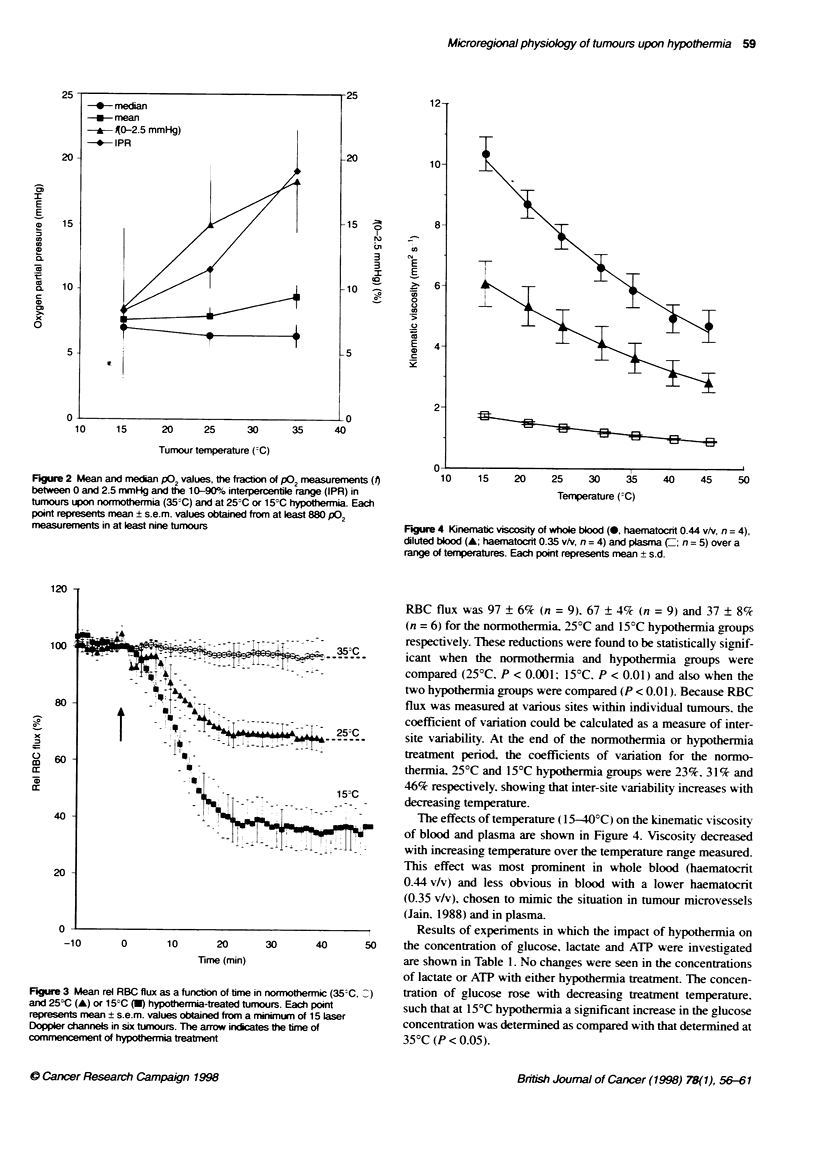
The effect of localized hypothermia on microcirculatory and metabolic parameters in s.c. DS sarcomas on the hind foot dorsum of Sprague-Dawley rats was investigated. Tumours were cooled by superfusion of the tumour surface with cooled saline solution to 25 degrees C or 15 degrees C. Control tumours remained at 35 degrees C. These temperatures were maintained for 30 min. In tumour oxygenation measurements, hypothermia at 25 degrees C and 15 degrees C caused progressive decreases in the size of the fraction of pO2 measurements between 0 and 2.5 mmHg together with a reduction in pO2 variability. No significant changes in median or mean pO2 or in the fraction of pO2 measurements between 0 and 5 mmHg, and 0 and 10 mmHg were observed. Using laser Doppler flowmetry, red blood cell flux was found to decrease significantly upon 25 degrees C or 15 degrees C hypothermia treatment to 67% and 37% of starting values respectively, whereas no significant changes were seen in control tumours over the whole observation period. Viscosity was measured in blood and plasma samples over a range of temperatures and was found to increase with decreasing temperature. Assessment of tumour glucose levels showed an increased concentration of glucose following 15 degrees C hypothermia, an observation consistent with a 'slowing down' of glycolysis. No changes in lactate or adenylate phosphate levels were observed. As a way of improving tumour oxygenation, localized hypothermia may therefore be a useful means of radiosensitization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brizel D. M., Scully S. P., Harrelson J. M., Layfield L. J., Bean J. M., Prosnitz L. R., Dewhirst M. W. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996 Mar 1;56(5):941–943. [PubMed] [Google Scholar]
- Bush R. S. Current status and treatment of localized disease and future aspects. Int J Radiat Oncol Biol Phys. 1984 Aug;10(8):1165–1174. doi: 10.1016/0360-3016(84)90312-2. [DOI] [PubMed] [Google Scholar]
- Bush R. S., Jenkin R. D., Allt W. E., Beale F. A., Bean H., Dembo A. J., Pringle J. F. Definitive evidence for hypoxic cells influencing cure in cancer therapy. Br J Cancer Suppl. 1978 Jun;3:302–306. [PMC free article] [PubMed] [Google Scholar]
- Dische S., Anderson P. J., Sealy R., Watson E. R. Carcinoma of the cervix--anaemia, radiotherapy and hyperbaric oxygen. Br J Radiol. 1983 Apr;56(664):251–255. doi: 10.1259/0007-1285-56-664-251. [DOI] [PubMed] [Google Scholar]
- Freitas I., Baronzio G. F. Tumor hypoxia, reoxygenation and oxygenation strategies: possible role in photodynamic therapy. J Photochem Photobiol B. 1991 Oct;11(1):3–30. doi: 10.1016/1011-1344(91)80264-i. [DOI] [PubMed] [Google Scholar]
- Graeber T. G., Osmanian C., Jacks T., Housman D. E., Koch C. J., Lowe S. W., Giaccia A. J. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996 Jan 4;379(6560):88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- Grote J., Süsskind R., Vaupel P. Oxygen diffusivity in tumor tissue (DS-carcinosarcoma) under temperature conditions within the range of 20--40 degrees C. Pflugers Arch. 1977 Nov 25;372(1):37–42. doi: 10.1007/BF00582204. [DOI] [PubMed] [Google Scholar]
- Hirst D. G. Oxygen delivery to tumors. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1271–1277. doi: 10.1016/0360-3016(86)90152-5. [DOI] [PubMed] [Google Scholar]
- Hockel M., Schlenger K., Aral B., Mitze M., Schaffer U., Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996 Oct 1;56(19):4509–4515. [PubMed] [Google Scholar]
- Höckel M., Knoop C., Schlenger K., Vorndran B., Baussmann E., Mitze M., Knapstein P. G., Vaupel P. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol. 1993 Jan;26(1):45–50. doi: 10.1016/0167-8140(93)90025-4. [DOI] [PubMed] [Google Scholar]
- Jain R. K. Determinants of tumor blood flow: a review. Cancer Res. 1988 May 15;48(10):2641–2658. [PubMed] [Google Scholar]
- Kelleher D. K., Mattheinsen U., Thews O., Vaupel P. Blood flow, oxygenation, and bioenergetic status of tumors after erythropoietin treatment in normal and anemic rats. Cancer Res. 1996 Oct 15;56(20):4728–4734. [PubMed] [Google Scholar]
- Kluge M., Elger B., Engel T., Schaefer C., Seega J., Vaupel P. Acute effects of tumor necrosis factor alpha or lymphotoxin on global blood flow, laser Doppler flux, and bioenergetic status of subcutaneous rodent tumors. Cancer Res. 1992 Apr 15;52(8):2167–2173. [PubMed] [Google Scholar]
- Konerding M. A., Steinberg F., Budach V. The vascular system of xenotransplanted tumors--scanning electron and light microscopic studies. Scanning Microsc. 1989 Mar;3(1):327–336. [PubMed] [Google Scholar]
- Nias A. H., Perry P. M., Photiou A. R. Modulating the oxygen tension in tumours by hypothermia and hyperbaric oxygen. J R Soc Med. 1988 Nov;81(11):633–636. doi: 10.1177/014107688808101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nias A. H., Perry P., Photiou A., Reghebi K. Effect of hypothermia on radiosensitization. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Aug;50(2):241–251. doi: 10.1080/09553008614550631. [DOI] [PubMed] [Google Scholar]
- Reeves R. B. The effect of temperature on the oxygen equilibrium curve of human blood. Respir Physiol. 1980 Dec;42(3):317–328. doi: 10.1016/0034-5687(80)90122-x. [DOI] [PubMed] [Google Scholar]
- Secomb T. W., Hsu R., Ong E. T., Gross J. F., Dewhirst M. W. Analysis of the effects of oxygen supply and demand on hypoxic fraction in tumors. Acta Oncol. 1995;34(3):313–316. doi: 10.3109/02841869509093981. [DOI] [PubMed] [Google Scholar]
- Smits G. J., Roman R. J., Lombard J. H. Evaluation of laser-Doppler flowmetry as a measure of tissue blood flow. J Appl Physiol (1985) 1986 Aug;61(2):666–672. doi: 10.1152/jappl.1986.61.2.666. [DOI] [PubMed] [Google Scholar]
- Thews O., Kelleher D. K., Vaupel P. In vivo oxygen consumption rate of DS sarcoma cells on inhibition of DNA synthesis. Cancer Res. 1996 May 1;56(9):2009–2012. [PubMed] [Google Scholar]
- Vaupel P. W. The influence of tumor blood flow and microenvironmental factors on the efficacy of radiation, drugs and localized hyperthermia. Klin Padiatr. 1997 Jul-Aug;209(4):243–249. doi: 10.1055/s-2008-1043957. [DOI] [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F. Physiological effects of hyperthermia. Recent Results Cancer Res. 1987;104:71–109. doi: 10.1007/978-3-642-82955-0_3. [DOI] [PubMed] [Google Scholar]
- Vaupel P., Schlenger K., Knoop C., Höckel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991 Jun 15;51(12):3316–3322. [PubMed] [Google Scholar]


