Abstract
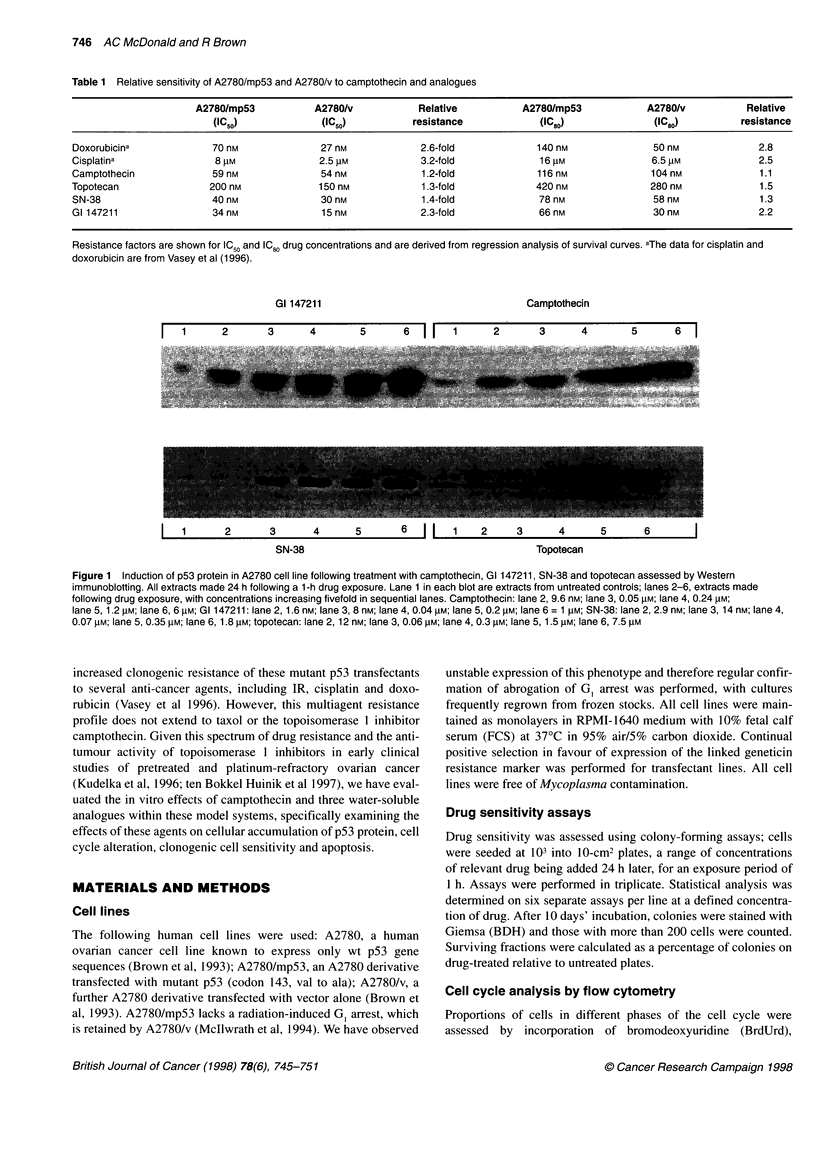
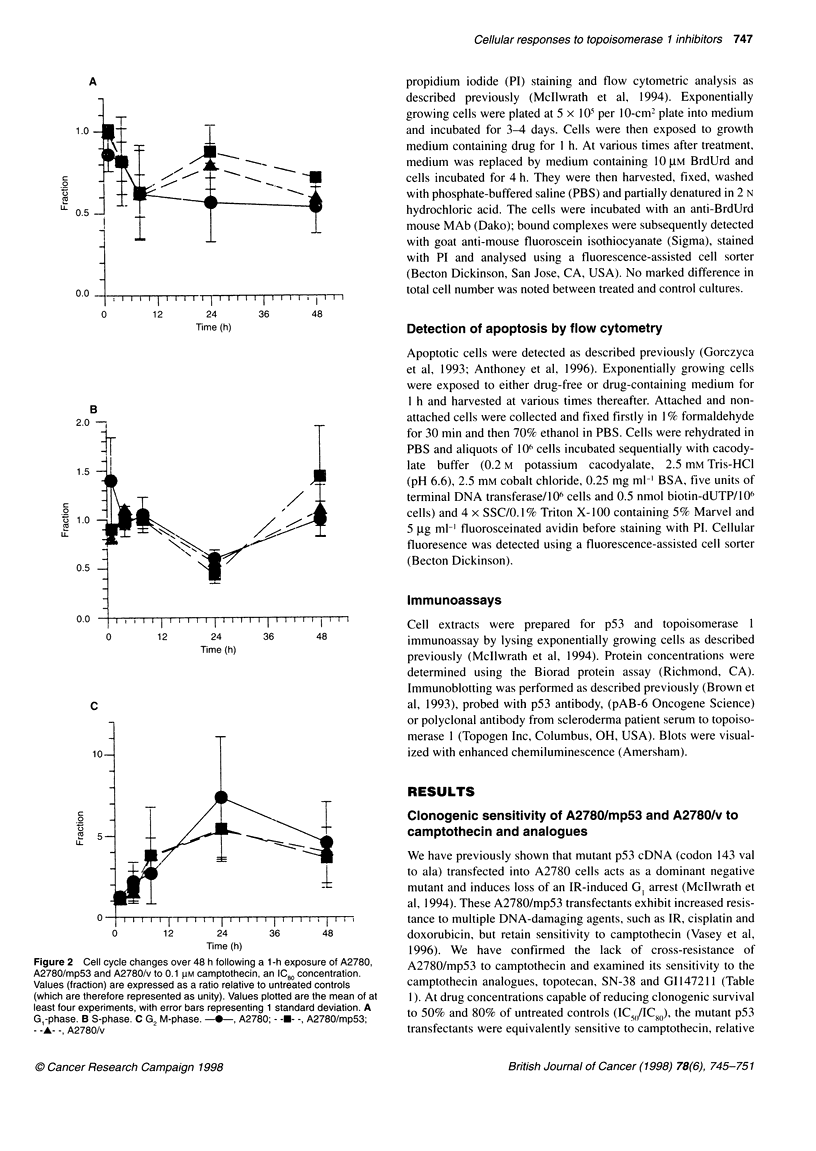
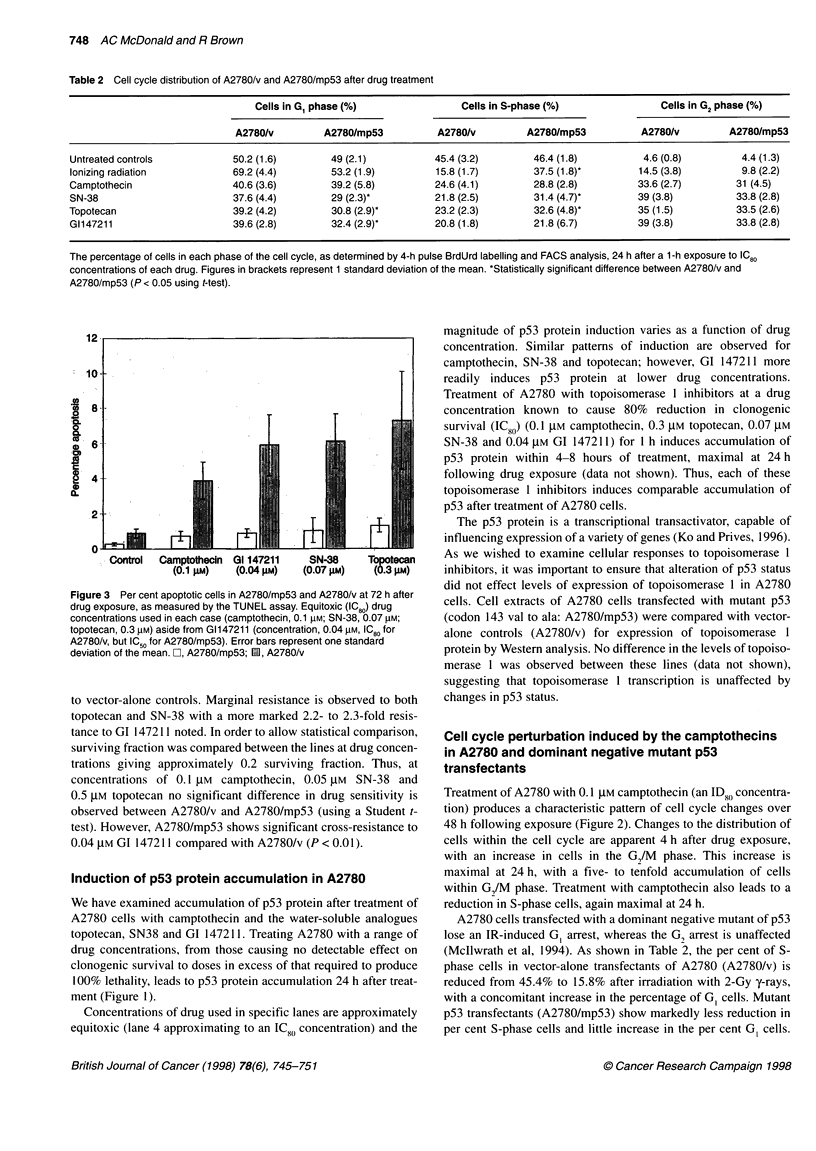
We have previously shown that loss of p53 function in A2780 human ovarian adenocarcinoma cells confers increased clonogenic resistance to several DNA-damaging agents, but not to taxol or camptothecin. We have now extended these studies, comparing wild-type p53-expressing A2780 cells with isogenic derivatives transfected with a dominant negative mutant (143; val to ala) p53. We show that, as well as retaining equivalent clonogenic sensitivity to camptothecin, mutant p53 transfectants of A2780 cells do not acquire significantly increased resistance to the camptothecin analogues topotecan and SN-38, the active metabolite of CPT-11. Compared with vector-alone transfectants they are, however, relatively (2.2-fold) resistant to GI 147211, a further camptothecin analogue undergoing clinical trial. Treatment of A2780 with camptothecin and each analogue produces an increase, maximal at 24-48 h after drug exposure, of cells in the G2/M phase of the cell cycle and a decrease in both G1 and S-phase cells. The G2 arrest is independent of p53 function for camptothecin and the three analogues. All four compounds can induce apoptosis in A2780, which is reduced in mutant p53 transfectants, as measured using the terminal DNA transferase-mediated b-d UTP nick end labelling (TUNEL) assay. Thus, although p53-dependent apoptosis is induced by camptothecin, topotecan and SN-38 in this human ovarian carcinoma cell line, these drugs induce p53-independent death, as measured by clonogenic assay.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal M. L., Agarwal A., Taylor W. R., Stark G. R. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthoney D. A., McIlwrath A. J., Gallagher W. M., Edlin A. R., Brown R. Microsatellite instability, apoptosis, and loss of p53 function in drug-resistant tumor cells. Cancer Res. 1996 Mar 15;56(6):1374–1381. [PubMed] [Google Scholar]
- Armand J. P., Ducreux M., Mahjoubi M., Abigerges D., Bugat R., Chabot G., Herait P., de Forni M., Rougier P. CPT-11 (irinotecan) in the treatment of colorectal cancer. Eur J Cancer. 1995 Jul-Aug;31A(7-8):1283–1287. doi: 10.1016/0959-8049(95)00212-2. [DOI] [PubMed] [Google Scholar]
- Barlogie B., Drewinko B. Cell cycle stage-dependent induction of G2 phase arrest by different antitumor agents. Eur J Cancer. 1978 Jul;14(7):741–745. doi: 10.1016/0014-2964(78)90002-6. [DOI] [PubMed] [Google Scholar]
- Brown R., Clugston C., Burns P., Edlin A., Vasey P., Vojtesek B., Kaye S. B. Increased accumulation of p53 protein in cisplatin-resistant ovarian cell lines. Int J Cancer. 1993 Oct 21;55(4):678–684. doi: 10.1002/ijc.2910550428. [DOI] [PubMed] [Google Scholar]
- Buttitta F., Marchetti A., Gadducci A., Pellegrini S., Morganti M., Carnicelli V., Cosio S., Gagetti O., Genazzani A. R., Bevilacqua G. p53 alterations are predictive of chemoresistance and aggressiveness in ovarian carcinomas: a molecular and immunohistochemical study. Br J Cancer. 1997;75(2):230–235. doi: 10.1038/bjc.1997.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter T. G., Glynn J. M., Echeverri F., Green D. R. The induction of apoptosis by chemotherapeutic agents occurs in all phases of the cell cycle. Anticancer Res. 1992 May-Jun;12(3):773–779. [PubMed] [Google Scholar]
- Creemers G. J., Bolis G., Gore M., Scarfone G., Lacave A. J., Guastalla J. P., Despax R., Favalli G., Kreinberg R., Van Belle S. Topotecan, an active drug in the second-line treatment of epithelial ovarian cancer: results of a large European phase II study. J Clin Oncol. 1996 Dec;14(12):3056–3061. doi: 10.1200/JCO.1996.14.12.3056. [DOI] [PubMed] [Google Scholar]
- Del Bino G., Skierski J. S., Darzynkiewicz Z. Diverse effects of camptothecin, an inhibitor of topoisomerase I, on the cell cycle of lymphocytic (L1210, MOLT-4) and myelogenous (HL-60, KG1) leukemic cells. Cancer Res. 1990 Sep 15;50(18):5746–5750. [PubMed] [Google Scholar]
- Fan S., el-Deiry W. S., Bae I., Freeman J., Jondle D., Bhatia K., Fornace A. J., Jr, Magrath I., Kohn K. W., O'Connor P. M. p53 gene mutations are associated with decreased sensitivity of human lymphoma cells to DNA damaging agents. Cancer Res. 1994 Nov 15;54(22):5824–5830. [PubMed] [Google Scholar]
- Fisher R. I. Report of workshop 5: early high-dose chemotherapy. Ann Oncol. 1996;7 (Suppl 4):131–133. doi: 10.1093/annonc/7.suppl_4.s131. [DOI] [PubMed] [Google Scholar]
- Fritsche M., Haessler C., Brandner G. Induction of nuclear accumulation of the tumor-suppressor protein p53 by DNA-damaging agents. Oncogene. 1993 Feb;8(2):307–318. [PubMed] [Google Scholar]
- Goldwasser F., Shimizu T., Jackman J., Hoki Y., O'Connor P. M., Kohn K. W., Pommier Y. Correlations between S and G2 arrest and the cytotoxicity of camptothecin in human colon carcinoma cells. Cancer Res. 1996 Oct 1;56(19):4430–4437. [PubMed] [Google Scholar]
- Gorczyca W., Gong J., Ardelt B., Traganos F., Darzynkiewicz Z. The cell cycle related differences in susceptibility of HL-60 cells to apoptosis induced by various antitumor agents. Cancer Res. 1993 Jul 1;53(13):3186–3192. [PubMed] [Google Scholar]
- Hamaguchi K., Godwin A. K., Yakushiji M., O'Dwyer P. J., Ozols R. F., Hamilton T. C. Cross-resistance to diverse drugs is associated with primary cisplatin resistance in ovarian cancer cell lines. Cancer Res. 1993 Nov 1;53(21):5225–5232. [PubMed] [Google Scholar]
- Hawkins D. S., Demers G. W., Galloway D. A. Inactivation of p53 enhances sensitivity to multiple chemotherapeutic agents. Cancer Res. 1996 Feb 15;56(4):892–898. [PubMed] [Google Scholar]
- Hsiang Y. H., Liu L. F. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988 Apr 1;48(7):1722–1726. [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Ko L. J., Prives C. p53: puzzle and paradigm. Genes Dev. 1996 May 1;10(9):1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- Kohn E. C., Sarosy G., Bicher A., Link C., Christian M., Steinberg S. M., Rothenberg M., Adamo D. O., Davis P., Ognibene F. P. Dose-intense taxol: high response rate in patients with platinum-resistant recurrent ovarian cancer. J Natl Cancer Inst. 1994 Jan 5;86(1):18–24. doi: 10.1093/jnci/86.1.18. [DOI] [PubMed] [Google Scholar]
- Kudelka A. P., Tresukosol D., Edwards C. L., Freedman R. S., Levenback C., Chantarawiroj P., Gonzalez de Leon C., Kim E. E., Madden T., Wallin B. Phase II study of intravenous topotecan as a 5-day infusion for refractory epithelial ovarian carcinoma. J Clin Oncol. 1996 May;14(5):1552–1557. doi: 10.1200/JCO.1996.14.5.1552. [DOI] [PubMed] [Google Scholar]
- Markman M., Rothman R., Hakes T., Reichman B., Hoskins W., Rubin S., Jones W., Almadrones L., Lewis J. L., Jr Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991 Mar;9(3):389–393. doi: 10.1200/JCO.1991.9.3.389. [DOI] [PubMed] [Google Scholar]
- McIlwrath A. J., Vasey P. A., Ross G. M., Brown R. Cell cycle arrests and radiosensitivity of human tumor cell lines: dependence on wild-type p53 for radiosensitivity. Cancer Res. 1994 Jul 15;54(14):3718–3722. [PubMed] [Google Scholar]
- Nelson W. G., Kastan M. B. DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol Cell Biol. 1994 Mar;14(3):1815–1823. doi: 10.1128/mcb.14.3.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor P. M., Jackman J., Jondle D., Bhatia K., Magrath I., Kohn K. W. Role of the p53 tumor suppressor gene in cell cycle arrest and radiosensitivity of Burkitt's lymphoma cell lines. Cancer Res. 1993 Oct 15;53(20):4776–4780. [PubMed] [Google Scholar]
- Pantazis P., Mendoza J. T., Early J. A., Kozielski A. J., Natelson E. A., Giovanella B. C. 9-Nitro-camptothecin delays growth of U-937 leukemia tumors in nude mice and is cytotoxic or cytostatic for human myelomonocytic leukemia lines in vitro. Eur J Haematol. 1993 Feb;50(2):81–89. doi: 10.1111/j.1600-0609.1993.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Perego P., Giarola M., Righetti S. C., Supino R., Caserini C., Delia D., Pierotti M. A., Miyashita T., Reed J. C., Zunino F. Association between cisplatin resistance and mutation of p53 gene and reduced bax expression in ovarian carcinoma cell systems. Cancer Res. 1996 Feb 1;56(3):556–562. [PubMed] [Google Scholar]
- Potmesil M. Camptothecins: from bench research to hospital wards. Cancer Res. 1994 Mar 15;54(6):1431–1439. [PubMed] [Google Scholar]
- Righetti S. C., Della Torre G., Pilotti S., Ménard S., Ottone F., Colnaghi M. I., Pierotti M. A., Lavarino C., Cornarotti M., Oriana S. A comparative study of p53 gene mutations, protein accumulation, and response to cisplatin-based chemotherapy in advanced ovarian carcinoma. Cancer Res. 1996 Feb 15;56(4):689–693. [PubMed] [Google Scholar]
- Shelling A. N., Cooke I. E., Ganesan T. S. The genetic analysis of ovarian cancer. Br J Cancer. 1995 Sep;72(3):521–527. doi: 10.1038/bjc.1995.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slichenmyer W. J., Nelson W. G., Slebos R. J., Kastan M. B. Loss of a p53-associated G1 checkpoint does not decrease cell survival following DNA damage. Cancer Res. 1993 Sep 15;53(18):4164–4168. [PubMed] [Google Scholar]
- Stewart N., Hicks G. G., Paraskevas F., Mowat M. Evidence for a second cell cycle block at G2/M by p53. Oncogene. 1995 Jan 5;10(1):109–115. [PubMed] [Google Scholar]
- Tsao Y. P., D'Arpa P., Liu L. F. The involvement of active DNA synthesis in camptothecin-induced G2 arrest: altered regulation of p34cdc2/cyclin B. Cancer Res. 1992 Apr 1;52(7):1823–1829. [PubMed] [Google Scholar]
- Vasey P. A., Jones N. A., Jenkins S., Dive C., Brown R. Cisplatin, camptothecin, and taxol sensitivities of cells with p53-associated multidrug resistance. Mol Pharmacol. 1996 Dec;50(6):1536–1540. [PubMed] [Google Scholar]
- Wahl A. F., Donaldson K. L., Fairchild C., Lee F. Y., Foster S. A., Demers G. W., Galloway D. A. Loss of normal p53 function confers sensitization to Taxol by increasing G2/M arrest and apoptosis. Nat Med. 1996 Jan;2(1):72–79. doi: 10.1038/nm0196-72. [DOI] [PubMed] [Google Scholar]
- Waldman T., Zhang Y., Dillehay L., Yu J., Kinzler K., Vogelstein B., Williams J. Cell-cycle arrest versus cell death in cancer therapy. Nat Med. 1997 Sep;3(9):1034–1036. doi: 10.1038/nm0997-1034. [DOI] [PubMed] [Google Scholar]
- ten Bokkel Huinink W., Gore M., Carmichael J., Gordon A., Malfetano J., Hudson I., Broom C., Scarabelli C., Davidson N., Spanczynski M. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol. 1997 Jun;15(6):2183–2193. doi: 10.1200/JCO.1997.15.6.2183. [DOI] [PubMed] [Google Scholar]



