Abstract
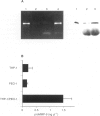
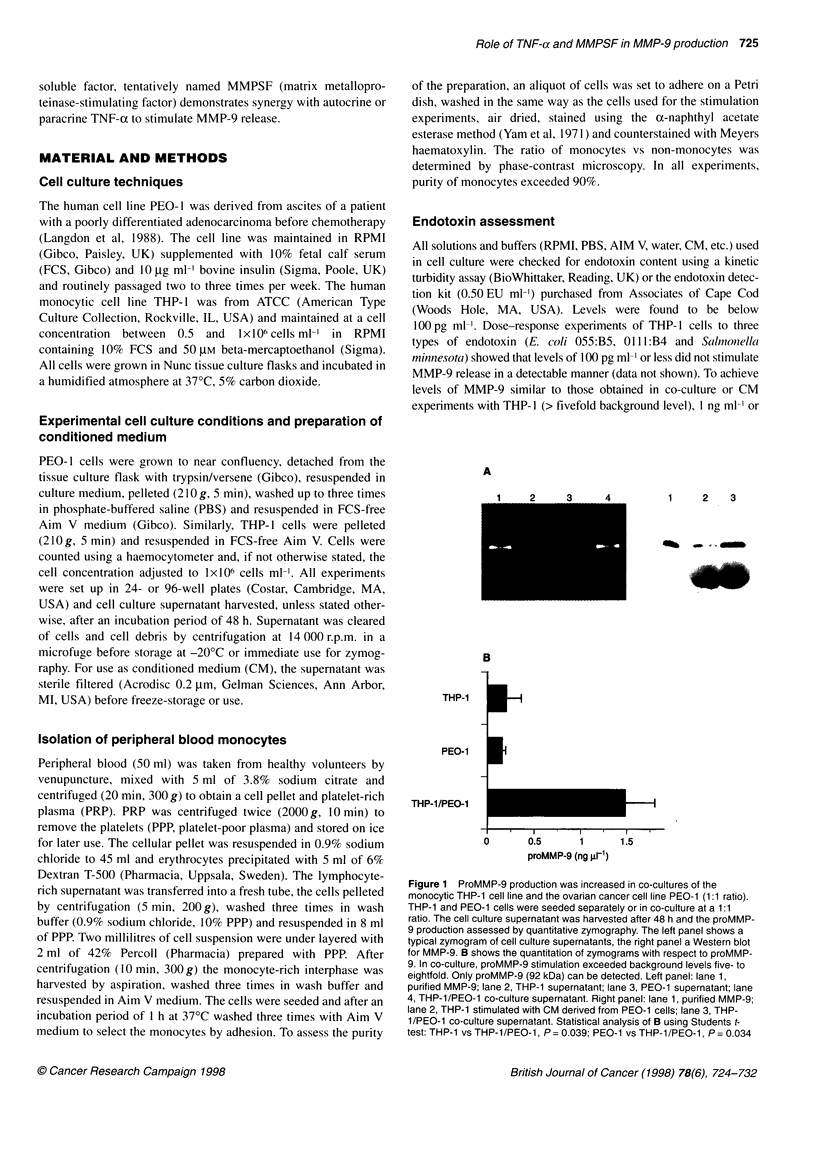
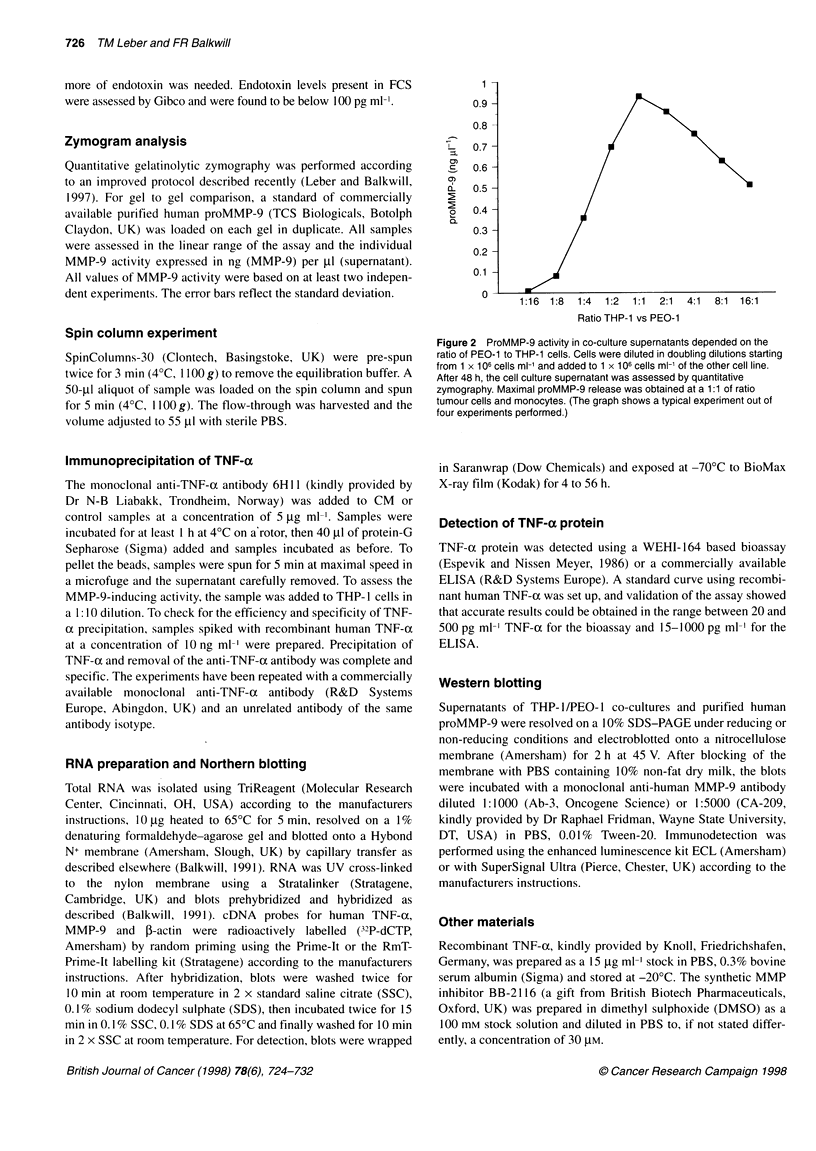
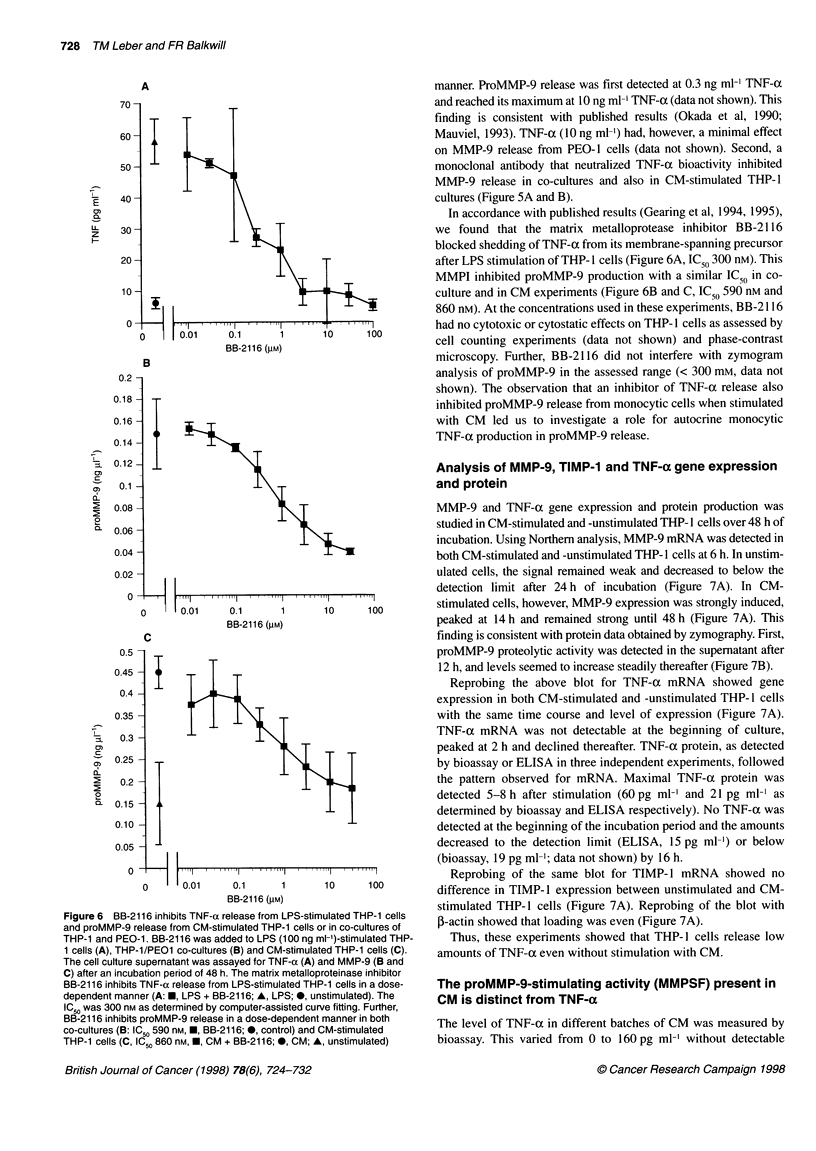
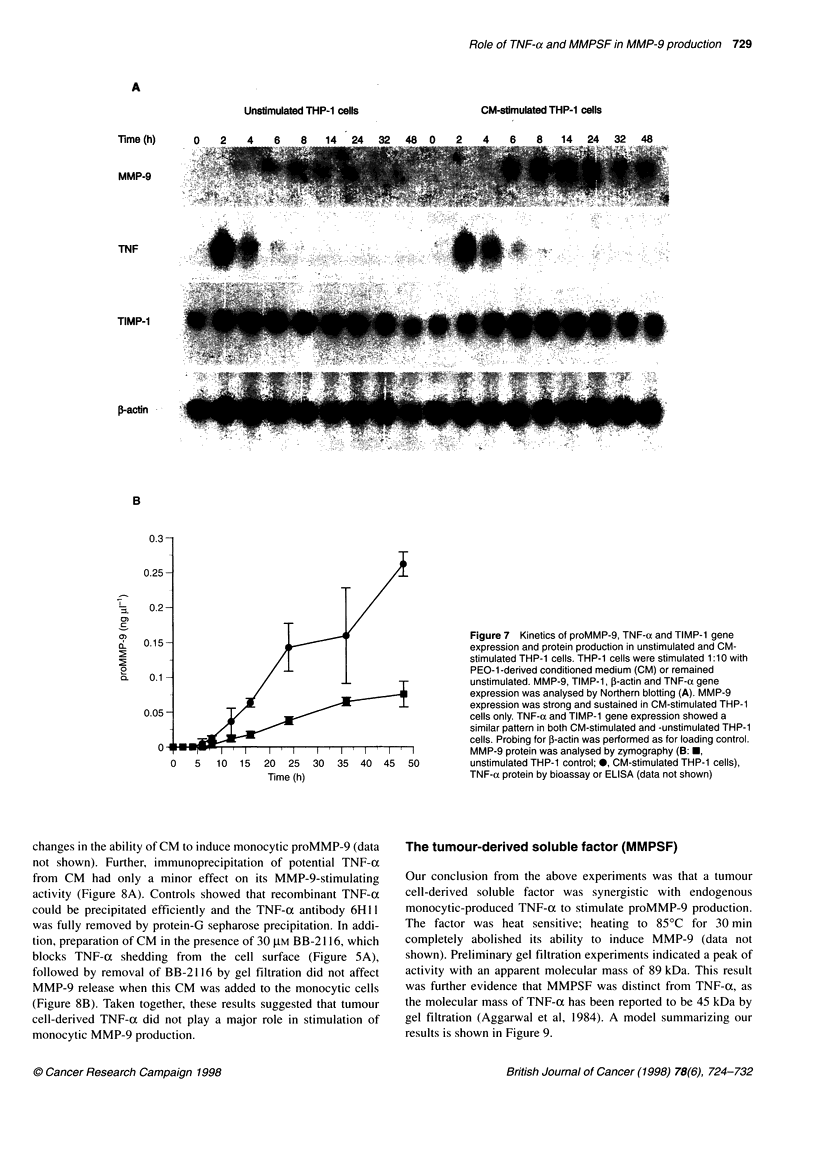
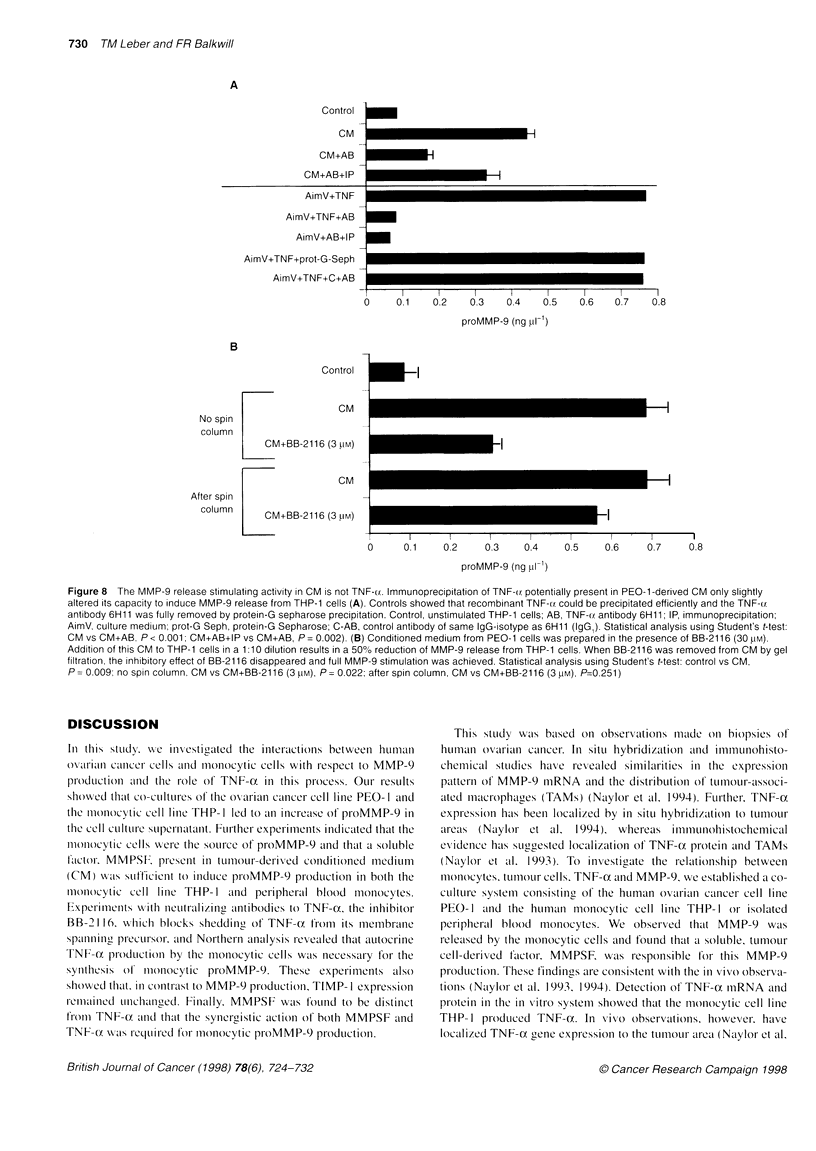
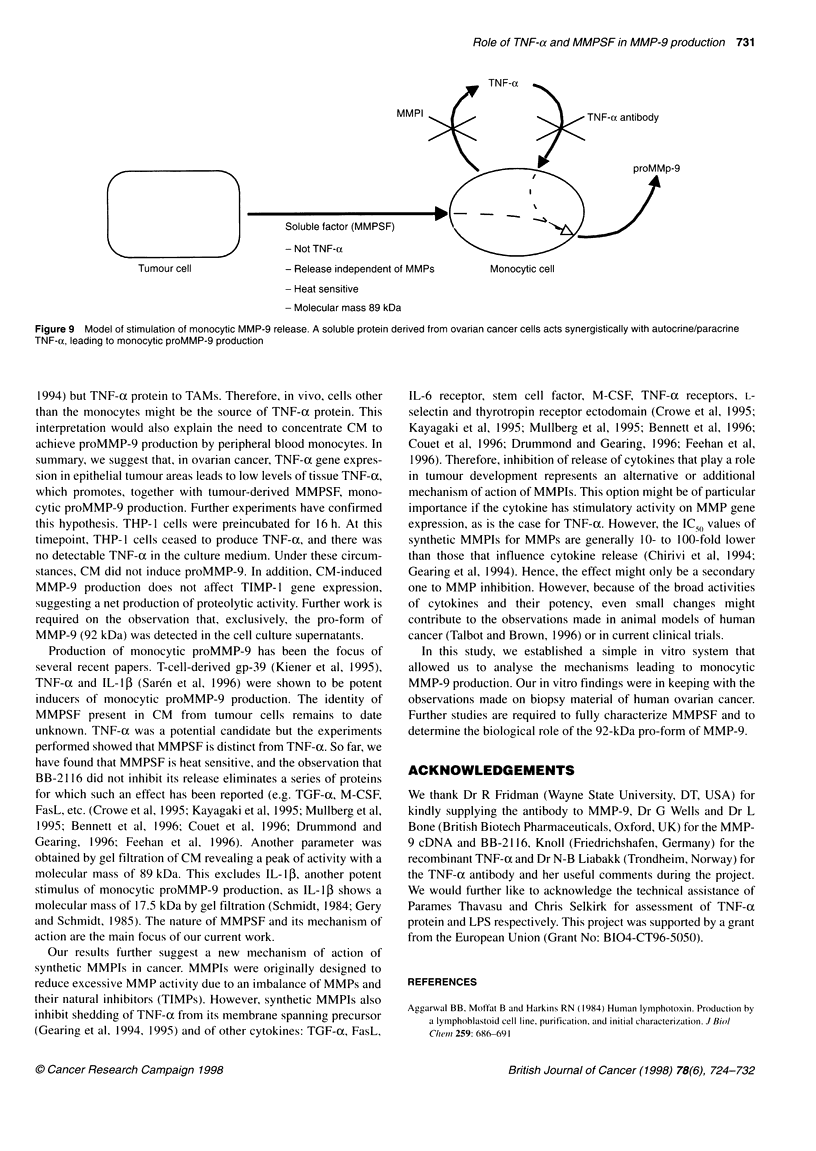
The matrix metalloprotease MMP-9 localizes to tumour-associated macrophages in human ovarian cancer but little is known of its regulation. Co-culture of human ovarian cancer cells (PEO-1) and a monocytic cell line (THP-1) led to production of 92-kDa proMMP-9. PEO-1-conditioned medium (CM) also stimulated THP-1 cells or isolated peripheral blood monocytes to produce proMMP-9. Expression of TIMP-1, however, remained unaffected. There was evidence that tumour necrosis factor alpha (TNF-alpha) was involved in tumour-stimulated monocytic proMMP-9 production. Antibody to TNF-alpha inhibited proMMP-9 production, and synthesis of TNF-alpha mRNA and protein preceded proMMP-9 release. In addition, the synthetic matrix metalloprotease inhibitor (MMPI) BB-2116, which blocks TNF-alpha shedding, inhibited proMMP-9 release in the co-cultures and from CM-stimulated monocytic cells. Further experiments suggested that the stimulating factor present in CM was not TNF-alpha, but acted synergistically with autocrine monocyte-derived TNF-alpha to release monocytic proMMP-9. Thus, ovarian cancer cells can stimulate monocytic cells in vitro to make proMMP-9 without affecting the expression of its inhibitor TIMP-1. This induction is mediated via a soluble factor (provisionally named MMPSF) that requires synergistic action of autocrine or paracrine TNF-alpha.
Full text
PDF
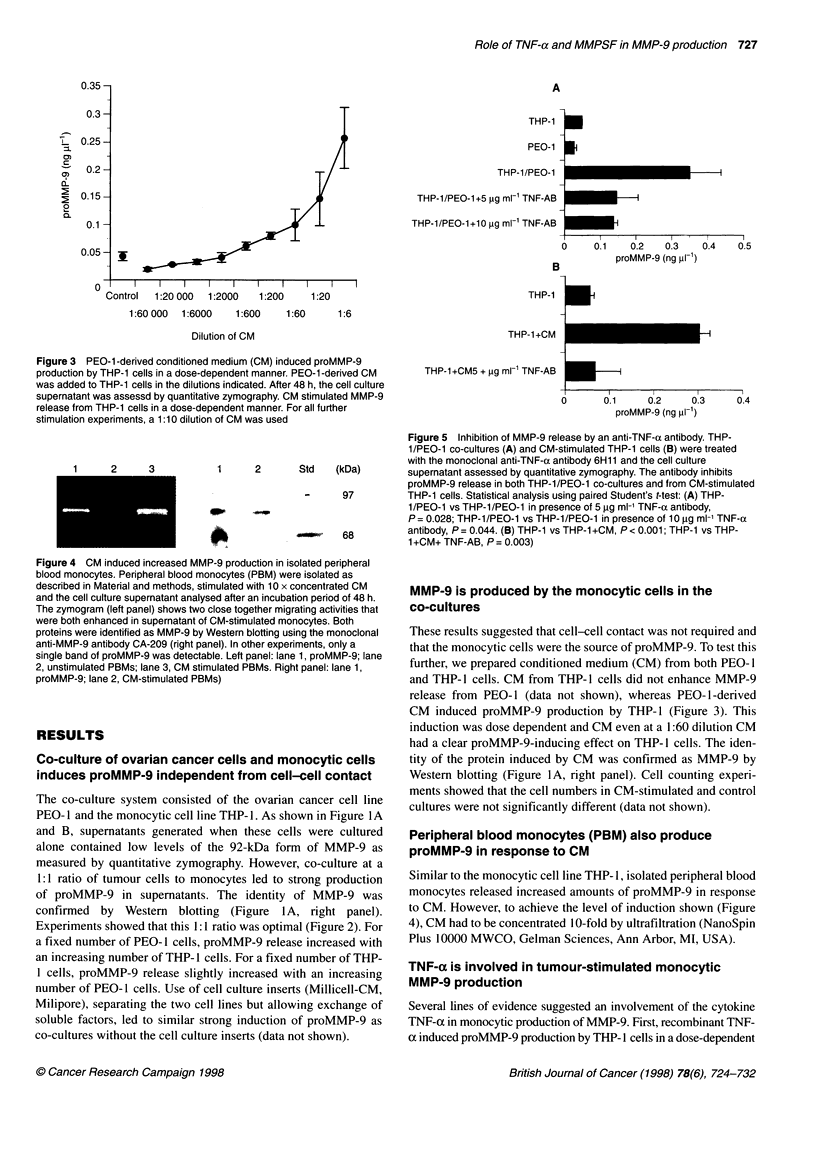

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Moffat B., Harkins R. N. Human lymphotoxin. Production by a lymphoblastoid cell line, purification, and initial characterization. J Biol Chem. 1984 Jan 10;259(1):686–691. [PubMed] [Google Scholar]
- Bennett T. A., Lynam E. B., Sklar L. A., Rogelj S. Hydroxamate-based metalloprotease inhibitor blocks shedding of L-selectin adhesion molecule from leukocytes: functional consequences for neutrophil aggregation. J Immunol. 1996 May 1;156(9):3093–3097. [PubMed] [Google Scholar]
- Bullen E. C., Longaker M. T., Updike D. L., Benton R., Ladin D., Hou Z., Howard E. W. Tissue inhibitor of metalloproteinases-1 is decreased and activated gelatinases are increased in chronic wounds. J Invest Dermatol. 1995 Feb;104(2):236–240. doi: 10.1111/1523-1747.ep12612786. [DOI] [PubMed] [Google Scholar]
- Chirivi R. G., Garofalo A., Crimmin M. J., Bawden L. J., Stoppacciaro A., Brown P. D., Giavazzi R. Inhibition of the metastatic spread and growth of B16-BL6 murine melanoma by a synthetic matrix metalloproteinase inhibitor. Int J Cancer. 1994 Aug 1;58(3):460–464. doi: 10.1002/ijc.2910580326. [DOI] [PubMed] [Google Scholar]
- Couet J., Sar S., Jolivet A., Hai M. T., Milgrom E., Misrahi M. Shedding of human thyrotropin receptor ectodomain. Involvement of a matrix metalloprotease. J Biol Chem. 1996 Feb 23;271(8):4545–4552. doi: 10.1074/jbc.271.8.4545. [DOI] [PubMed] [Google Scholar]
- Crowe P. D., Walter B. N., Mohler K. M., Otten-Evans C., Black R. A., Ware C. F. A metalloprotease inhibitor blocks shedding of the 80-kD TNF receptor and TNF processing in T lymphocytes. J Exp Med. 1995 Mar 1;181(3):1205–1210. doi: 10.1084/jem.181.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B., Brown P. D., East N., Crimmin M. J., Balkwill F. R. A synthetic matrix metalloproteinase inhibitor decreases tumor burden and prolongs survival of mice bearing human ovarian carcinoma xenografts. Cancer Res. 1993 May 1;53(9):2087–2091. [PubMed] [Google Scholar]
- Davies B., Waxman J., Wasan H., Abel P., Williams G., Krausz T., Neal D., Thomas D., Hanby A., Balkwill F. Levels of matrix metalloproteases in bladder cancer correlate with tumor grade and invasion. Cancer Res. 1993 Nov 15;53(22):5365–5369. [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Feehan C., Darlak K., Kahn J., Walcheck B., Spatola A. F., Kishimoto T. K. Shedding of the lymphocyte L-selectin adhesion molecule is inhibited by a hydroxamic acid-based protease inhibitor. Identification with an L-selectin-alkaline phosphatase reporter. J Biol Chem. 1996 Mar 22;271(12):7019–7024. doi: 10.1074/jbc.271.12.7019. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Beckett P., Christodoulou M., Churchill M., Clements J. M., Crimmin M., Davidson A. H., Drummond A. H., Galloway W. A., Gilbert R. Matrix metalloproteinases and processing of pro-TNF-alpha. J Leukoc Biol. 1995 May;57(5):774–777. doi: 10.1002/jlb.57.5.774. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Beckett P., Christodoulou M., Churchill M., Clements J., Davidson A. H., Drummond A. H., Galloway W. A., Gilbert R., Gordon J. L. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994 Aug 18;370(6490):555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- Gery I., Schmidt J. A. Human interleukin 1. Methods Enzymol. 1985;116:456–467. doi: 10.1016/s0076-6879(85)16037-4. [DOI] [PubMed] [Google Scholar]
- Hamdy F. C., Fadlon E. J., Cottam D., Lawry J., Thurrell W., Silcocks P. B., Anderson J. B., Williams J. L., Rees R. C. Matrix metalloproteinase 9 expression in primary human prostatic adenocarcinoma and benign prostatic hyperplasia. Br J Cancer. 1994 Jan;69(1):177–182. doi: 10.1038/bjc.1994.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina T. V., Goldberg G. I., Eisen A. Z. Matrix metalloproteinases in blood vessel development in human fetal skin and in cutaneous tumors. J Invest Dermatol. 1995 Sep;105(3):411–417. doi: 10.1111/1523-1747.ep12321097. [DOI] [PubMed] [Google Scholar]
- Kayagaki N., Kawasaki A., Ebata T., Ohmoto H., Ikeda S., Inoue S., Yoshino K., Okumura K., Yagita H. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995 Dec 1;182(6):1777–1783. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiener P. A., Moran-Davis P., Rankin B. M., Wahl A. F., Aruffo A., Hollenbaugh D. Stimulation of CD40 with purified soluble gp39 induces proinflammatory responses in human monocytes. J Immunol. 1995 Nov 15;155(10):4917–4925. [PubMed] [Google Scholar]
- Leber T. M., Balkwill F. R. Zymography: a single-step staining method for quantitation of proteolytic activity on substrate gels. Anal Biochem. 1997 Jun 15;249(1):24–28. doi: 10.1006/abio.1997.2170. [DOI] [PubMed] [Google Scholar]
- Liabakk N. B., Talbot I., Smith R. A., Wilkinson K., Balkwill F. Matrix metalloprotease 2 (MMP-2) and matrix metalloprotease 9 (MMP-9) type IV collagenases in colorectal cancer. Cancer Res. 1996 Jan 1;56(1):190–196. [PubMed] [Google Scholar]
- Liotta L. A., Stetler-Stevenson W. G. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991 Sep 15;51(18 Suppl):5054s–5059s. [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Mauch C., Krieg T., Bauer E. A. Role of the extracellular matrix in the degradation of connective tissue. Arch Dermatol Res. 1994;287(1):107–114. doi: 10.1007/BF00370728. [DOI] [PubMed] [Google Scholar]
- Mauviel A. Cytokine regulation of metalloproteinase gene expression. J Cell Biochem. 1993 Dec;53(4):288–295. doi: 10.1002/jcb.240530404. [DOI] [PubMed] [Google Scholar]
- Murphy G. Matrix metalloproteinases and their inhibitors. Acta Orthop Scand Suppl. 1995 Oct;266:55–60. [PubMed] [Google Scholar]
- Müllberg J., Durie F. H., Otten-Evans C., Alderson M. R., Rose-John S., Cosman D., Black R. A., Mohler K. M. A metalloprotease inhibitor blocks shedding of the IL-6 receptor and the p60 TNF receptor. J Immunol. 1995 Dec 1;155(11):5198–5205. [PubMed] [Google Scholar]
- Naylor M. S., Stamp G. W., Davies B. D., Balkwill F. R. Expression and activity of MMPS and their regulators in ovarian cancer. Int J Cancer. 1994 Jul 1;58(1):50–56. doi: 10.1002/ijc.2910580110. [DOI] [PubMed] [Google Scholar]
- Naylor M. S., Stamp G. W., Foulkes W. D., Eccles D., Balkwill F. R. Tumor necrosis factor and its receptors in human ovarian cancer. Potential role in disease progression. J Clin Invest. 1993 May;91(5):2194–2206. doi: 10.1172/JCI116446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Tsuchiya H., Shimizu H., Tomita K., Nakanishi I., Sato H., Seiki M., Yamashita K., Hayakawa T. Induction and stimulation of 92-kDa gelatinase/type IV collagenase production in osteosarcoma and fibrosarcoma cell lines by tumor necrosis factor alpha. Biochem Biophys Res Commun. 1990 Sep 14;171(2):610–617. doi: 10.1016/0006-291x(90)91190-4. [DOI] [PubMed] [Google Scholar]
- Sang Q. X., Birkedal-Hansen H., Van Wart H. E. Proteolytic and non-proteolytic activation of human neutrophil progelatinase B. Biochim Biophys Acta. 1995 Sep 6;1251(2):99–108. doi: 10.1016/0167-4838(95)00086-a. [DOI] [PubMed] [Google Scholar]
- Sarén P., Welgus H. G., Kovanen P. T. TNF-alpha and IL-1beta selectively induce expression of 92-kDa gelatinase by human macrophages. J Immunol. 1996 Nov 1;157(9):4159–4165. [PubMed] [Google Scholar]
- Schmidt J. A. Purification and partial biochemical characterization of normal human interleukin 1. J Exp Med. 1984 Sep 1;160(3):772–787. doi: 10.1084/jem.160.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G. Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am J Pathol. 1996 May;148(5):1345–1350. [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr, Gunja-Smith Z. Role of metalloproteinases in human osteoarthritis. J Rheumatol Suppl. 1991 Feb;27:99–101. [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]