Abstract
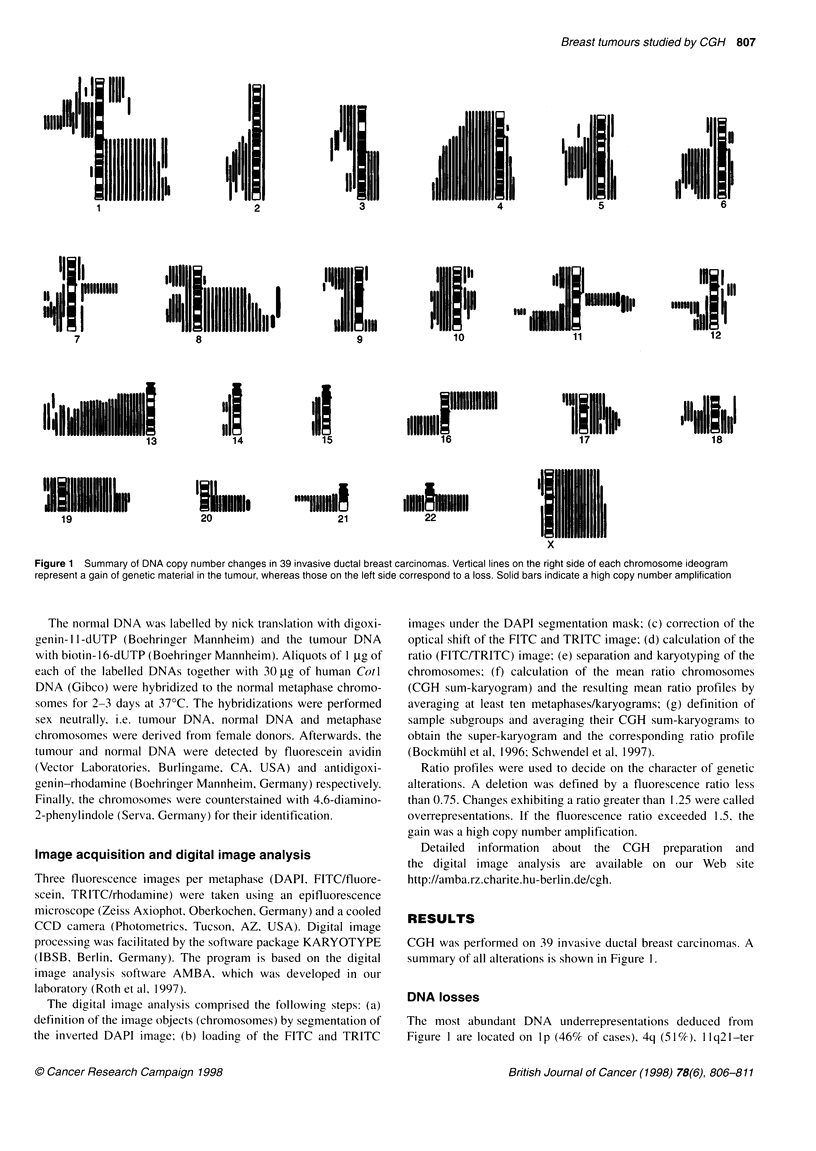
Comparative genomic hybridization was applied to map DNA gains and losses in 39 invasive ductal breast carcinomas. Frequent abnormalities included gains on chromosomal regions 1q, 8q, 11q12-13, 16p, 19, 20q and X as well as frequent losses on 1p, 5q, 6q, 9p, 11q, 13q and 16q. Furthermore, frequent losses on 4q (20 cases) and 21q (14 cases) were found for the first time in this tumour type. High copy number amplifications were observed at 8q12-24, 11q11-13 and 20q13-ter. Highly differentiated tumours were associated with gains on 1q and 11q12-13 along with losses on 1p21-22, 4q, 13q, 11q21-ter. Undifferentiated breast carcinomas were characterized by additional DNA imbalances, i.e. deletions of 5q13-23, all of chromosome 9, the centromeric part of chromosome 13 including band 13q14 and the overrepresentation of chromosome X. We speculate that these changes are associated with tumour progression of invasive ductal breast cancer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen T. I., Gaustad A., Ottestad L., Farrants G. W., Nesland J. M., Tveit K. M., Børresen A. L. Genetic alterations of the tumour suppressor gene regions 3p, 11p, 13q, 17p, and 17q in human breast carcinomas. Genes Chromosomes Cancer. 1992 Mar;4(2):113–121. doi: 10.1002/gcc.2870040203. [DOI] [PubMed] [Google Scholar]
- Anzick S. L., Kononen J., Walker R. L., Azorsa D. O., Tanner M. M., Guan X. Y., Sauter G., Kallioniemi O. P., Trent J. M., Meltzer P. S. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997 Aug 15;277(5328):965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- BLOOM H. J., RICHARDSON W. W. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957 Sep;11(3):359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkqvist A. M., Tammilehto L., Anttila S., Mattson K., Knuutila S. Recurrent DNA copy number changes in 1q, 4q, 6q, 9p, 13q, 14q and 22q detected by comparative genomic hybridization in malignant mesothelioma. Br J Cancer. 1997;75(4):523–527. doi: 10.1038/bjc.1997.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland K. I., Konstadoulakis M. M., Vezeridis M. P., Wanebo H. J. Oncogene protein co-expression. Value of Ha-ras, c-myc, c-fos, and p53 as prognostic discriminants for breast carcinoma. Ann Surg. 1995 Jun;221(6):706–720. doi: 10.1097/00000658-199506000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockmühl U., Schwendel A., Dietel M., Petersen I. Distinct patterns of chromosomal alterations in high- and low-grade head and neck squamous cell carcinomas. Cancer Res. 1996 Dec 1;56(23):5325–5329. [PubMed] [Google Scholar]
- Brison O. Gene amplification and tumor progression. Biochim Biophys Acta. 1993 May 25;1155(1):25–41. doi: 10.1016/0304-419x(93)90020-d. [DOI] [PubMed] [Google Scholar]
- Clark G. M., McGuire W. L. Follow-up study of HER-2/neu amplification in primary breast cancer. Cancer Res. 1991 Feb 1;51(3):944–948. [PubMed] [Google Scholar]
- Cliby W., Ritland S., Hartmann L., Dodson M., Halling K. C., Keeney G., Podratz K. C., Jenkins R. B. Human epithelial ovarian cancer allelotype. Cancer Res. 1993 May 15;53(10 Suppl):2393–2398. [PubMed] [Google Scholar]
- Courjal F., Louason G., Speiser P., Katsaros D., Zeillinger R., Theillet C. Cyclin gene amplification and overexpression in breast and ovarian cancers: evidence for the selection of cyclin D1 in breast and cyclin E in ovarian tumors. Int J Cancer. 1996 Aug 22;69(4):247–253. doi: 10.1002/(SICI)1097-0215(19960822)69:4<247::AID-IJC1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Courjal F., Theillet C. Comparative genomic hybridization analysis of breast tumors with predetermined profiles of DNA amplification. Cancer Res. 1997 Oct 1;57(19):4368–4377. [PubMed] [Google Scholar]
- Cox L. A., Chen G., Lee E. Y. Tumor suppressor genes and their roles in breast cancer. Breast Cancer Res Treat. 1994;32(1):19–38. doi: 10.1007/BF00666203. [DOI] [PubMed] [Google Scholar]
- Cunningham J. M., Ingle J. N., Jung S. H., Cha S. S., Wold L. E., Farr G., Witzig T. E., Krook J. E., Wieand H. S., Kovach J. S. p53 gene expression in node-positive breast cancer: relationship to DNA ploidy and prognosis. J Natl Cancer Inst. 1994 Dec 21;86(24):1871–1873. doi: 10.1093/jnci/86.24.1871. [DOI] [PubMed] [Google Scholar]
- Devilee P., van Vliet M., van Sloun P., Kuipers Dijkshoorn N., Hermans J., Pearson P. L., Cornelisse C. J. Allelotype of human breast carcinoma: a second major site for loss of heterozygosity is on chromosome 6q. Oncogene. 1991 Sep;6(9):1705–1711. [PubMed] [Google Scholar]
- Eeles R. A., Stratton M. R., Goldgar D. E., Easton D. F. The genetics of familial breast cancer and their practical implications. Eur J Cancer. 1994;30A(9):1383–1390. doi: 10.1016/0959-8049(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Groden J., Thliveris A., Samowitz W., Carlson M., Gelbert L., Albertsen H., Joslyn G., Stevens J., Spirio L., Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991 Aug 9;66(3):589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- Guan X. Y., Xu J., Anzick S. L., Zhang H., Trent J. M., Meltzer P. S. Hybrid selection of transcribed sequences from microdissected DNA: isolation of genes within amplified region at 20q11-q13.2 in breast cancer. Cancer Res. 1996 Aug 1;56(15):3446–3450. [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994 Nov 18;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Isola J. J., Kallioniemi O. P., Chu L. W., Fuqua S. A., Hilsenbeck S. G., Osborne C. K., Waldman F. M. Genetic aberrations detected by comparative genomic hybridization predict outcome in node-negative breast cancer. Am J Pathol. 1995 Oct;147(4):905–911. [PMC free article] [PubMed] [Google Scholar]
- Isola J., DeVries S., Chu L., Ghazvini S., Waldman F. Analysis of changes in DNA sequence copy number by comparative genomic hybridization in archival paraffin-embedded tumor samples. Am J Pathol. 1994 Dec;145(6):1301–1308. [PMC free article] [PubMed] [Google Scholar]
- Kallioniemi A., Kallioniemi O. P., Piper J., Tanner M., Stokke T., Chen L., Smith H. S., Pinkel D., Gray J. W., Waldman F. M. Detection and mapping of amplified DNA sequences in breast cancer by comparative genomic hybridization. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2156–2160. doi: 10.1073/pnas.91.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallioniemi A., Kallioniemi O. P., Sudar D., Rutovitz D., Gray J. W., Waldman F., Pinkel D. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science. 1992 Oct 30;258(5083):818–821. doi: 10.1126/science.1359641. [DOI] [PubMed] [Google Scholar]
- Kelley M. J., Nakagawa K., Steinberg S. M., Mulshine J. L., Kamb A., Johnson B. E. Differential inactivation of CDKN2 and Rb protein in non-small-cell and small-cell lung cancer cell lines. J Natl Cancer Inst. 1995 May 17;87(10):756–761. doi: 10.1093/jnci/87.10.756. [DOI] [PubMed] [Google Scholar]
- Kerangueven F., Allione F., Noguchi T., Adélaïde J., Sobol H., Jacquemier J., Birnbaum D. Patterns of loss of heterozygosity at loci from chromosome arm 13q suggests a possible involvement of BRCA2 in sporadic breast tumors. Genes Chromosomes Cancer. 1995 Aug;13(4):291–294. doi: 10.1002/gcc.2870130410. [DOI] [PubMed] [Google Scholar]
- Koreth J., Bakkenist C. J., McGee J. O. Allelic deletions at chromosome 11q22-q23.1 and 11q25-qterm are frequent in sporadic breast but not colorectal cancers. Oncogene. 1997 Jan 30;14(4):431–437. doi: 10.1038/sj.onc.1200847. [DOI] [PubMed] [Google Scholar]
- Kuukasjärvi T., Karhu R., Tanner M., Kähkönen M., Schäffer A., Nupponen N., Pennanen S., Kallioniemi A., Kallioniemi O. P., Isola J. Genetic heterogeneity and clonal evolution underlying development of asynchronous metastasis in human breast cancer. Cancer Res. 1997 Apr 15;57(8):1597–1604. [PubMed] [Google Scholar]
- Laake K., Odegård A., Andersen T. I., Bukholm I. K., Kåresen R., Nesland J. M., Ottestad L., Shiloh Y., Børresen-Dale A. L. Loss of heterozygosity at 11q23.1 in breast carcinomas: indication for involvement of a gene distal and close to ATM. Genes Chromosomes Cancer. 1997 Mar;18(3):175–180. [PubMed] [Google Scholar]
- Lancaster J. M., Wooster R., Mangion J., Phelan C. M., Cochran C., Gumbs C., Seal S., Barfoot R., Collins N., Bignell G. BRCA2 mutations in primary breast and ovarian cancers. Nat Genet. 1996 Jun;13(2):238–240. doi: 10.1038/ng0696-238. [DOI] [PubMed] [Google Scholar]
- Marchio A., Meddeb M., Pineau P., Danglot G., Tiollais P., Bernheim A., Dejean A. Recurrent chromosomal abnormalities in hepatocellular carcinoma detected by comparative genomic hybridization. Genes Chromosomes Cancer. 1997 Jan;18(1):59–65. [PubMed] [Google Scholar]
- Nishisho I., Nakamura Y., Miyoshi Y., Miki Y., Ando H., Horii A., Koyama K., Utsunomiya J., Baba S., Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991 Aug 9;253(5020):665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Norton A. J., Jordan S., Yeomans P. Brief, high-temperature heat denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed tissues. J Pathol. 1994 Aug;173(4):371–379. doi: 10.1002/path.1711730413. [DOI] [PubMed] [Google Scholar]
- Polascik T. J., Cairns P., Epstein J. I., Fuzesi L., Ro J. Y., Marshall F. F., Sidransky D., Schoenberg M. Distal nephron renal tumors: microsatellite allelotype. Cancer Res. 1996 Apr 15;56(8):1892–1895. [PubMed] [Google Scholar]
- Ried T., Just K. E., Holtgreve-Grez H., du Manoir S., Speicher M. R., Schröck E., Latham C., Blegen H., Zetterberg A., Cremer T. Comparative genomic hybridization of formalin-fixed, paraffin-embedded breast tumors reveals different patterns of chromosomal gains and losses in fibroadenomas and diploid and aneuploid carcinomas. Cancer Res. 1995 Nov 15;55(22):5415–5423. [PubMed] [Google Scholar]
- Rimm D. L., Sinard J. H., Morrow J. S. Reduced alpha-catenin and E-cadherin expression in breast cancer. Lab Invest. 1995 May;72(5):506–512. [PubMed] [Google Scholar]
- Roth K., Wolf G., Dietel M., Petersen I. Image analysis for comparative genomic hybridization based on a karyotyping program for windows. Anal Quant Cytol Histol. 1997 Dec;19(6):461–474. [PubMed] [Google Scholar]
- Sakata K., Tamura G., Nishizuka S., Maesawa C., Suzuki Y., Iwaya T., Terashima M., Saito K., Satodate R. Commonly deleted regions on the long arm of chromosome 21 in differentiated adenocarcinoma of the stomach. Genes Chromosomes Cancer. 1997 Apr;18(4):318–321. [PubMed] [Google Scholar]
- Schmutzler R. K., Fimmers R., Bierhoff E., Lohmar B., Homann A., Speiser P., Kubista E., Jaeger K., Krebs D., Zeillinger R. Association of allelic losses on human chromosomal arms 11Q and 16Q in sporadic breast cancer. Int J Cancer. 1996 Aug 22;69(4):307–311. doi: 10.1002/(SICI)1097-0215(19960822)69:4<307::AID-IJC12>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Schwendel A., Langreck H., Reichel M., Schröck E., Ried T., Dietel M., Petersen I. Primary small-cell lung carcinomas and their metastases are characterized by a recurrent pattern of genetic alterations. Int J Cancer. 1997 Feb 20;74(1):86–93. doi: 10.1002/(sici)1097-0215(19970220)74:1<86::aid-ijc15>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Solinas-Toldo S., Wallrapp C., Müller-Pillasch F., Bentz M., Gress T., Lichter P. Mapping of chromosomal imbalances in pancreatic carcinoma by comparative genomic hybridization. Cancer Res. 1996 Aug 15;56(16):3803–3807. [PubMed] [Google Scholar]
- Spandidos D. A., Karaiossifidi H., Malliri A., Linardopoulos S., Vassilaros S., Tsikkinis A., Field J. K. Expression of ras Rb1 and p53 proteins in human breast cancer. Anticancer Res. 1992 Jan-Feb;12(1):81–89. [PubMed] [Google Scholar]
- Tamura G., Sakata K., Nishizuka S., Maesawa C., Suzuki Y., Terashima M., Eda Y., Satodate R. Allelotype of adenoma and differentiated adenocarcinoma of the stomach. J Pathol. 1996 Dec;180(4):371–377. doi: 10.1002/(SICI)1096-9896(199612)180:4<371::AID-PATH704>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Tanner M. M., Tirkkonen M., Kallioniemi A., Collins C., Stokke T., Karhu R., Kowbel D., Shadravan F., Hintz M., Kuo W. L. Increased copy number at 20q13 in breast cancer: defining the critical region and exclusion of candidate genes. Cancer Res. 1994 Aug 15;54(16):4257–4260. [PubMed] [Google Scholar]
- Tirkkonen M., Johannsson O., Agnarsson B. A., Olsson H., Ingvarsson S., Karhu R., Tanner M., Isola J., Barkardottir R. B., Borg A. Distinct somatic genetic changes associated with tumor progression in carriers of BRCA1 and BRCA2 germ-line mutations. Cancer Res. 1997 Apr 1;57(7):1222–1227. [PubMed] [Google Scholar]
- Tsuda H., Callen D. F., Fukutomi T., Nakamura Y., Hirohashi S. Allele loss on chromosome 16q24.2-qter occurs frequently in breast cancers irrespectively of differences in phenotype and extent of spread. Cancer Res. 1994 Jan 15;54(2):513–517. [PubMed] [Google Scholar]
- Tsuda H., Hirohashi S., Shimosato Y., Hirota T., Tsugane S., Yamamoto H., Miyajima N., Toyoshima K., Yamamoto T., Yokota J. Correlation between long-term survival in breast cancer patients and amplification of two putative oncogene-coamplification units: hst-1/int-2 and c-erbB-2/ear-1. Cancer Res. 1989 Jun 1;49(11):3104–3108. [PubMed] [Google Scholar]
- Wasmuth J. J., Park C., Ferrell R. E. Report of the committee on the genetic constitution of chromosome 5. Cytogenet Cell Genet. 1989;51(1-4):137–148. doi: 10.1159/000132789. [DOI] [PubMed] [Google Scholar]
- Winqvist R., Hampton G. M., Mannermaa A., Blanco G., Alavaikko M., Kiviniemi H., Taskinen P. J., Evans G. A., Wright F. A., Newsham I. Loss of heterozygosity for chromosome 11 in primary human breast tumors is associated with poor survival after metastasis. Cancer Res. 1995 Jun 15;55(12):2660–2664. [PubMed] [Google Scholar]
- Wooster R., Bignell G., Lancaster J., Swift S., Seal S., Mangion J., Collins N., Gregory S., Gumbs C., Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995 Dec 21;378(6559):789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]




