Abstract
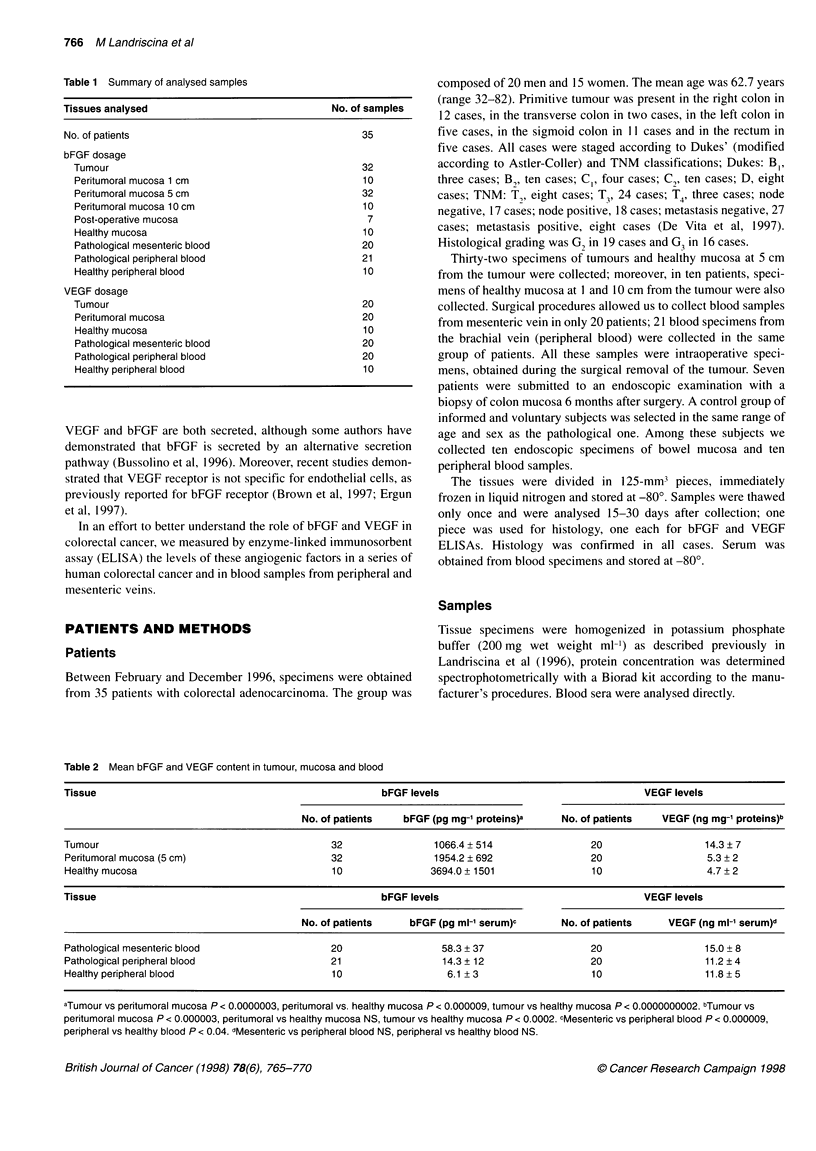
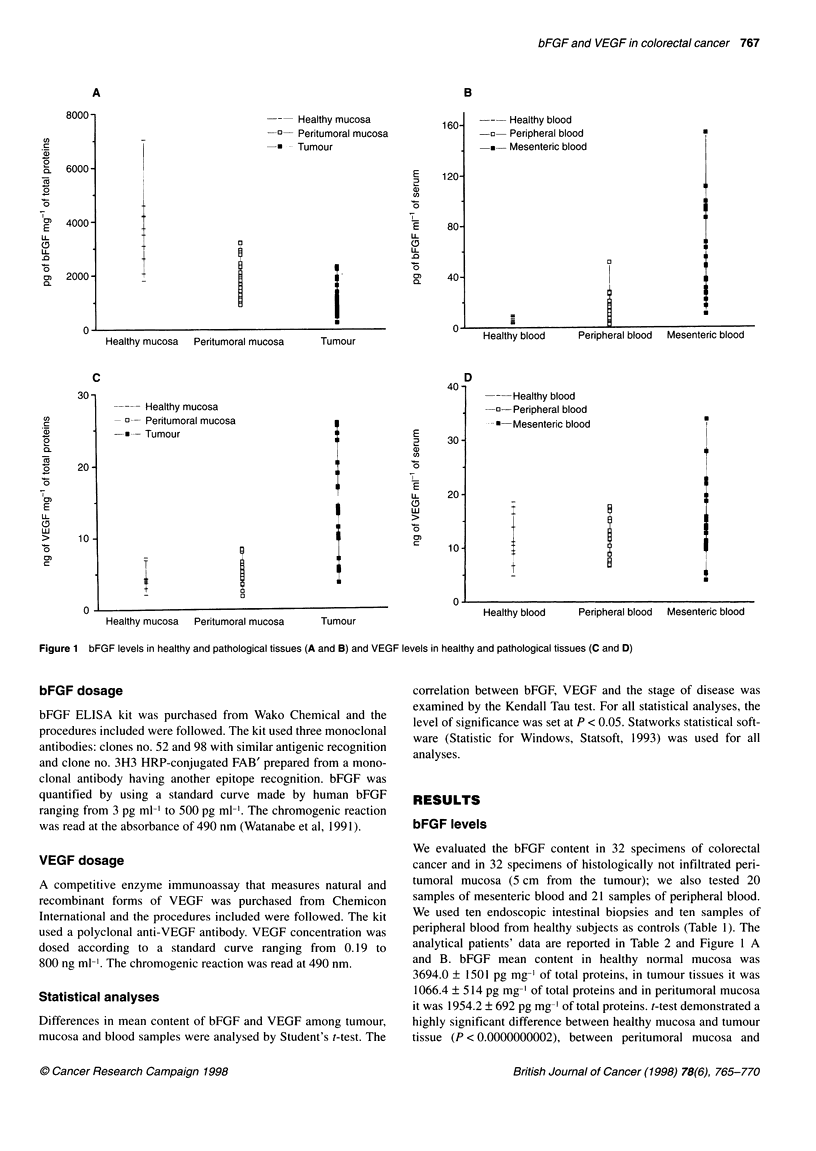
Tumour growth is angiogenesis dependent. Some authors suggest a prognostic role of microvessel count in colorectal cancer. We tested the role of basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) in the switch to the angiogenic phenotype in 35 patients with colorectal cancer at different stages of disease. We evaluated the two angiogenic factors, by enzyme-linked immunosorbent assay (ELISA), in tumour, peritumoral mucosa, pathological mesenteric and peripheral blood. We used ten endoscopic intestinal biopsies and ten peripheral blood samples from healthy subjects as control. bFGF was significantly lower in tumour tissues and in peritumoral mucosas than in healthy mucosas, whereas VEGF was up-regulated in tumours but not in peritumoral mucosa. Both angiogenic factors were greatly increased in mesenteric blood. VEGF tumour and serum levels were significantly correlated with the stage of disease. bFGF tumour and serum concentration were not correlated with the stage of disease. The high levels of bFGF in mesenteric blood suggest that this growth factor might be abnormally released from tumour tissue and peritumoral mucosa and could function as an early effector in the switch to the angiogenic phenotype. In contrast, VEGF, whose levels show a significant correlation with the stage of disease, could act in a following step, supporting tumour progression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basilico C., Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- Bossi P., Viale G., Lee A. K., Alfano R., Coggi G., Bosari S. Angiogenesis in colorectal tumors: microvessel quantitation in adenomas and carcinomas with clinicopathological correlations. Cancer Res. 1995 Nov 1;55(21):5049–5053. [PubMed] [Google Scholar]
- Brown L. F., Detmar M., Tognazzi K., Abu-Jawdeh G., Iruela-Arispe M. L. Uterine smooth muscle cells express functional receptors (flt-1 and KDR) for vascular permeability factor/vascular endothelial growth factor. Lab Invest. 1997 Feb;76(2):245–255. [PubMed] [Google Scholar]
- Bussolino F., Albini A., Camussi G., Presta M., Viglietto G., Ziche M., Persico G. Role of soluble mediators in angiogenesis. Eur J Cancer. 1996 Dec;32A(14):2401–2412. doi: 10.1016/s0959-8049(96)00390-5. [DOI] [PubMed] [Google Scholar]
- Dirix L. Y., Vermeulen P. B., Hubens G., Benoy I., Martin M., De Pooter C., Van Oosterom A. T. Serum basic fibroblast growth factor and vascular endothelial growth factor and tumour growth kinetics in advanced colorectal cancer. Ann Oncol. 1996 Oct;7(8):843–848. doi: 10.1093/oxfordjournals.annonc.a010764. [DOI] [PubMed] [Google Scholar]
- Ergün S., Kiliç N., Fiedler W., Mukhopadhyay A. K. Vascular endothelial growth factor and its receptors in normal human testicular tissue. Mol Cell Endocrinol. 1997 Jul 4;131(1):9–20. doi: 10.1016/s0303-7207(97)00082-8. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995 Jan;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Frank R. E., Saclarides T. J., Leurgans S., Speziale N. J., Drab E. A., Rubin D. B. Tumor angiogenesis as a predictor of recurrence and survival in patients with node-negative colon cancer. Ann Surg. 1995 Dec;222(6):695–699. doi: 10.1097/00000658-199512000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto F., Goto K., Weindel K., Folkman J. Synergistic effects of vascular endothelial growth factor and basic fibroblast growth factor on the proliferation and cord formation of bovine capillary endothelial cells within collagen gels. Lab Invest. 1993 Nov;69(5):508–517. [PubMed] [Google Scholar]
- Graham C. H., Rivers J., Kerbel R. S., Stankiewicz K. S., White W. L. Extent of vascularization as a prognostic indicator in thin (< 0.76 mm) malignant melanomas. Am J Pathol. 1994 Sep;145(3):510–514. [PMC free article] [PubMed] [Google Scholar]
- Kandel J., Bossy-Wetzel E., Radvanyi F., Klagsbrun M., Folkman J., Hanahan D. Neovascularization is associated with a switch to the export of bFGF in the multistep development of fibrosarcoma. Cell. 1991 Sep 20;66(6):1095–1104. doi: 10.1016/0092-8674(91)90033-u. [DOI] [PubMed] [Google Scholar]
- Kang S. M., Maeda K., Onoda N., Chung Y. S., Nakata B., Nishiguchi Y., Sowa M. Combined analysis of p53 and vascular endothelial growth factor expression in colorectal carcinoma for determination of tumor vascularity and liver metastasis. Int J Cancer. 1997 Oct 21;74(5):502–507. doi: 10.1002/(sici)1097-0215(19971021)74:5<502::aid-ijc4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Landriscina M., Remiddi F., Ria F., Palazzotti B., De Leo M. E., Iacoangeli M., Rosselli R., Scerrati M., Galeotti T. The level of MnSOD is directly correlated with grade of brain tumours of neuroepithelial origin. Br J Cancer. 1996 Dec;74(12):1877–1885. doi: 10.1038/bjc.1996.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindmark G., Gerdin B., Sundberg C., Påhlman L., Bergström R., Glimelius B. Prognostic significance of the microvascular count in colorectal cancer. J Clin Oncol. 1996 Feb;14(2):461–466. doi: 10.1200/JCO.1996.14.2.461. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D., Tsiokas L., Sukhatme V. P. Wild-type p53 and v-Src exert opposing influences on human vascular endothelial growth factor gene expression. Cancer Res. 1995 Dec 15;55(24):6161–6165. [PubMed] [Google Scholar]
- Smith-McCune K. K., Weidner N. Demonstration and characterization of the angiogenic properties of cervical dysplasia. Cancer Res. 1994 Feb 1;54(3):800–804. [PubMed] [Google Scholar]
- Takahashi Y., Kitadai Y., Bucana C. D., Cleary K. R., Ellis L. M. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995 Sep 15;55(18):3964–3968. [PubMed] [Google Scholar]
- Takahashi Y., Tucker S. L., Kitadai Y., Koura A. N., Bucana C. D., Cleary K. R., Ellis L. M. Vessel counts and expression of vascular endothelial growth factor as prognostic factors in node-negative colon cancer. Arch Surg. 1997 May;132(5):541–546. doi: 10.1001/archsurg.1997.01430290087018. [DOI] [PubMed] [Google Scholar]
- Tanigawa N., Amaya H., Matsumura M., Lu C., Kitaoka A., Matsuyama K., Muraoka R. Tumor angiogenesis and mode of metastasis in patients with colorectal cancer. Cancer Res. 1997 Mar 15;57(6):1043–1046. [PubMed] [Google Scholar]
- Vermeulen P. B., Verhoeven D., Fierens H., Hubens G., Goovaerts G., Van Marck E., De Bruijn E. A., Van Oosterom A. T., Dirix L. Y. Microvessel quantification in primary colorectal carcinoma: an immunohistochemical study. Br J Cancer. 1995 Feb;71(2):340–343. doi: 10.1038/bjc.1995.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Bar-Shavit R., Ishai-Michaeli R., Bashkin P., Fuks Z. Extracellular sequestration and release of fibroblast growth factor: a regulatory mechanism? Trends Biochem Sci. 1991 Jul;16(7):268–271. doi: 10.1016/0968-0004(91)90102-2. [DOI] [PubMed] [Google Scholar]
- Warren R. S., Yuan H., Matli M. R., Gillett N. A., Ferrara N. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest. 1995 Apr;95(4):1789–1797. doi: 10.1172/JCI117857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner N., Carroll P. R., Flax J., Blumenfeld W., Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993 Aug;143(2):401–409. [PMC free article] [PubMed] [Google Scholar]
- Weidner N., Folkman J., Pozza F., Bevilacqua P., Allred E. N., Moore D. H., Meli S., Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992 Dec 16;84(24):1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]


