Abstract
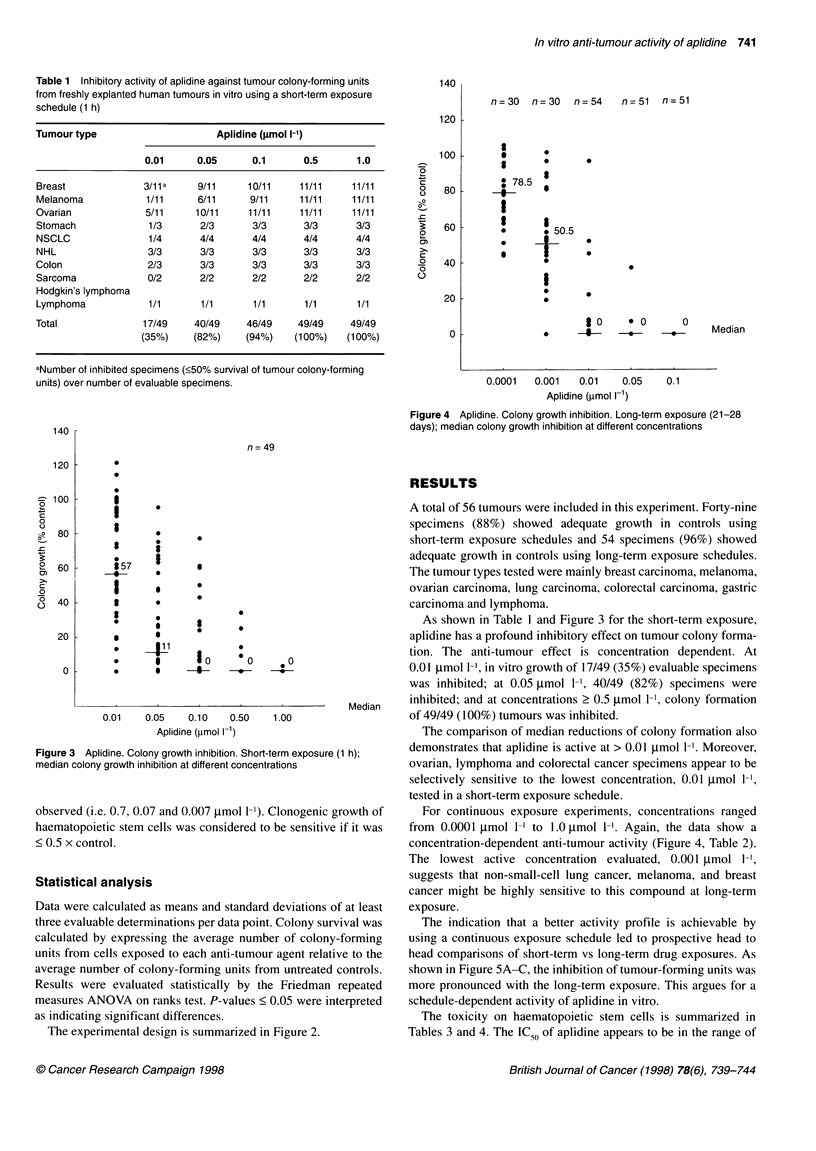
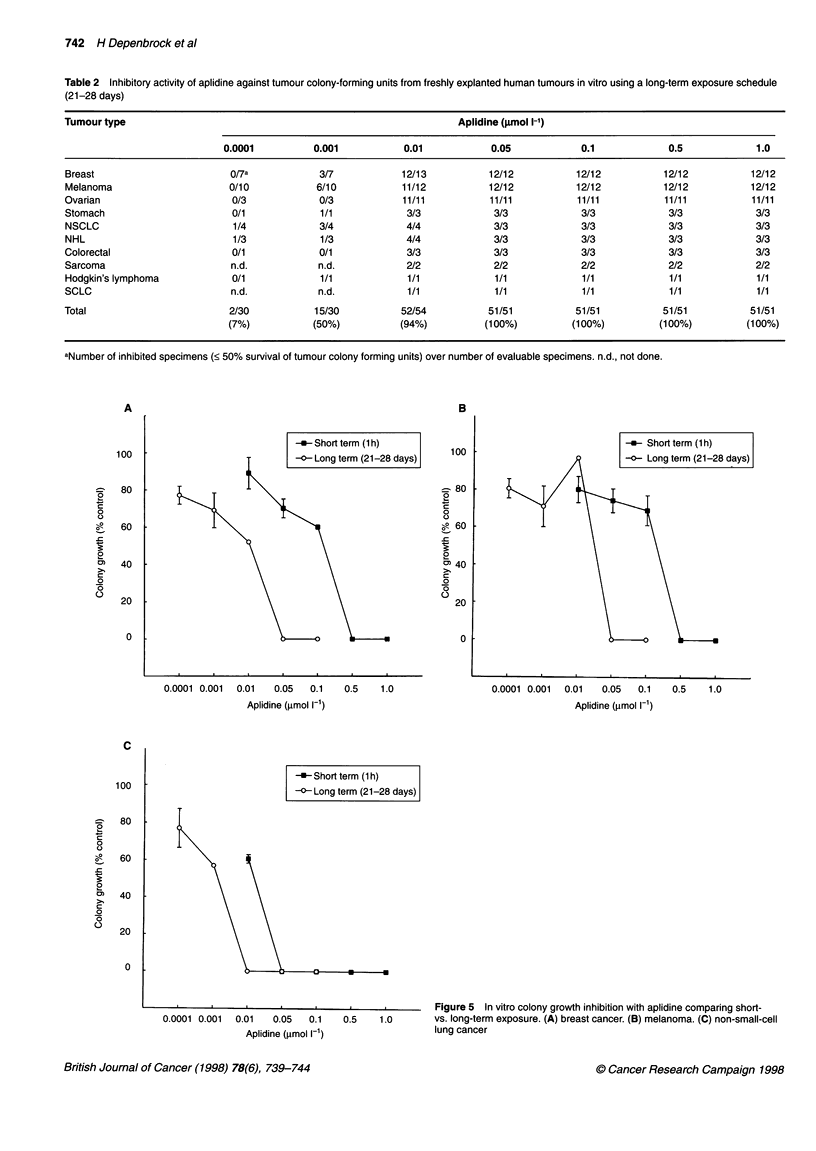
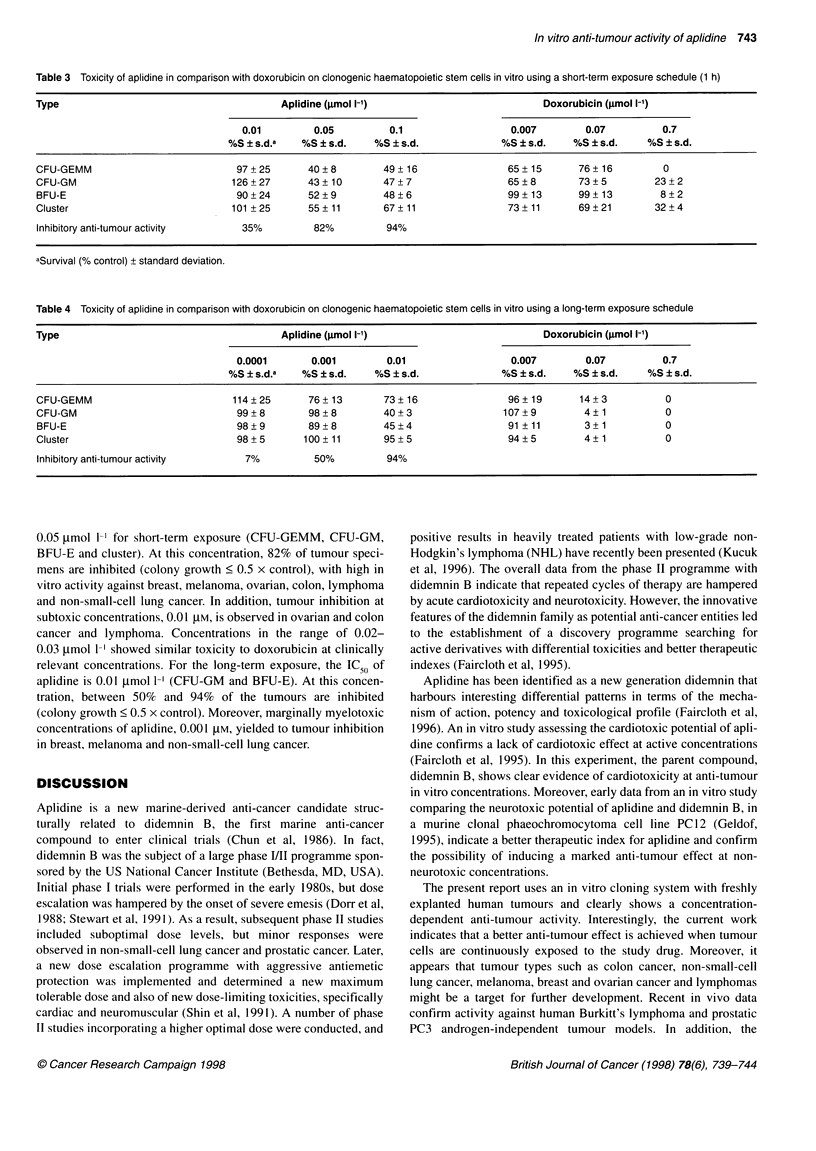
Aplidine is a new marine anti-cancer depsipeptide isolated from the Mediterranean tunicate Aplidium albicans. We have evaluated its antiproliferative action against a variety of freshly explanted human tumour specimens. Concentration ranges of 0.01-1.0 microM and 0.0001-1.0 microM were used in short- and long-term exposure schedules respectively. After exposure for 1 h in 49 evaluable specimens, aplidine showed a clear concentration-dependent anti-tumour effect. At 0.05 microM, 85% of the specimens were markedly inhibited. Continuous exposure for 21-28 days in 54 tumour specimens also led to a concentration-dependent activity relationship. Fifty per cent and 100% tumour inhibitions were achieved with 0.001 microM and 0.05 microM respectively. A head to head evaluation assessing short vs continuous exposure was carried out, resulting in evidence of an activity-time of exposure relationship. Breast, melanoma and non-small-cell lung cancer appear to be sensitive to low concentrations of aplidine. In addition the evaluation of the effects of aplidine on haematopoietic cells showed a concentration-dependent toxicity. However, under continuous exposure, active concentrations induced mild bone marrow toxicity, indicating that a therapeutic window at marginally myelotoxic concentrations might exist.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auvinen M. Cell transformation, invasion, and angiogenesis: a regulatory role for ornithine decarboxylase and polyamines? J Natl Cancer Inst. 1997 Apr 16;89(8):533–537. doi: 10.1093/jnci/89.8.533. [DOI] [PubMed] [Google Scholar]
- Bradley T. R. Aspects of stimulation of bone marrow colony growth in vitro. Aust J Exp Biol Med Sci. 1968 Jun;46(3):335–342. doi: 10.1038/icb.1968.27. [DOI] [PubMed] [Google Scholar]
- Chun H. G., Davies B., Hoth D., Suffness M., Plowman J., Flora K., Grieshaber C., Leyland-Jones B. Didemnin B. The first marine compound entering clinical trials as an antineoplastic agent. Invest New Drugs. 1986;4(3):279–284. doi: 10.1007/BF00179597. [DOI] [PubMed] [Google Scholar]
- Crews C. M., Collins J. L., Lane W. S., Snapper M. L., Schreiber S. L. GTP-dependent binding of the antiproliferative agent didemnin to elongation factor 1 alpha. J Biol Chem. 1994 Jun 3;269(22):15411–15414. [PubMed] [Google Scholar]
- Crews C. M., Lane W. S., Schreiber S. L. Didemnin binds to the protein palmitoyl thioesterase responsible for infantile neuronal ceroid lipofuscinosis. Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):4316–4319. doi: 10.1073/pnas.93.9.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr F. A., Kuhn J. G., Phillips J., von Hoff D. D. Phase I clinical and pharmacokinetic investigation of didemnin B, a cyclic depsipeptide. Eur J Cancer Clin Oncol. 1988 Nov;24(11):1699–1706. doi: 10.1016/0277-5379(88)90070-3. [DOI] [PubMed] [Google Scholar]
- Geldof A. A. Nerve-growth-factor-dependent neurite outgrowth assay; a research model for chemotherapy-induced neuropathy. J Cancer Res Clin Oncol. 1995;121(11):657–660. doi: 10.1007/BF01218523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Fabre P. M., de Pedro E., Medina M. A., Núez de Castro I., Márquez J. Polyamine contents of human breast cancer cells treated with the cytotoxic agents chlorpheniramine and dehydrodidemnin B. Cancer Lett. 1997 Feb 26;113(1-2):141–144. doi: 10.1016/s0304-3835(96)04591-0. [DOI] [PubMed] [Google Scholar]
- Hanauske U., Hanauske A. R., Marshall M. H., Muggia V. A., Von Hoff D. D. Biphasic effect of vanadium salts on in vitro tumor colony growth. Int J Cell Cloning. 1987 Mar;5(2):170–178. doi: 10.1002/stem.5530050209. [DOI] [PubMed] [Google Scholar]
- Lobo C., García-Pozo S. G., Núez de Castro I., Alonso F. J. Effect of dehydrodidemnin B on human colon carcinoma cell lines. Anticancer Res. 1997 Jan-Feb;17(1A):333–336. [PubMed] [Google Scholar]
- Malfetano J. H., Blessing J. A., Jacobs A. J. A phase II trial of Didemnin B (NSC #335319) in patients with previously treated epithelial ovarian cancer. A Gynecologic Oncology Group Study. Am J Clin Oncol. 1993 Feb;16(1):47–49. doi: 10.1097/00000421-199302000-00012. [DOI] [PubMed] [Google Scholar]
- Sakai R., Rinehart K. L., Kishore V., Kundu B., Faircloth G., Gloer J. B., Carney J. R., Namikoshi M., Sun F., Hughes R. G., Jr Structure--activity relationships of the didemnins. J Med Chem. 1996 Jul 5;39(14):2819–2834. doi: 10.1021/jm960048g. [DOI] [PubMed] [Google Scholar]
- Shin D. M., Holoye P. Y., Murphy W. K., Forman A., Papasozomenos S. C., Hong W. K., Raber M. Phase I/II clinical trial of didemnin B in non-small-cell lung cancer: neuromuscular toxicity is dose-limiting. Cancer Chemother Pharmacol. 1991;29(2):145–149. doi: 10.1007/BF00687325. [DOI] [PubMed] [Google Scholar]
- Stewart J. A., Low J. B., Roberts J. D., Blow A. A phase I clinical trial of didemnin B. Cancer. 1991 Dec 15;68(12):2550–2554. doi: 10.1002/1097-0142(19911215)68:12<2550::aid-cncr2820681203>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Urdiales J. L., Morata P., Núez De Castro I., Sánchez-Jiménez F. Antiproliferative effect of dehydrodidemnin B (DDB), a depsipeptide isolated from Mediterranean tunicates. Cancer Lett. 1996 Apr 19;102(1-2):31–37. doi: 10.1016/0304-3835(96)04151-1. [DOI] [PubMed] [Google Scholar]
- Von Hoff D. D., Forseth B. J., Huong M., Buchok J. B., Lathan B. Improved plating efficiencies for human tumors cloned in capillary tubes versus Petri dishes. Cancer Res. 1986 Aug;46(8):4012–4017. [PubMed] [Google Scholar]



