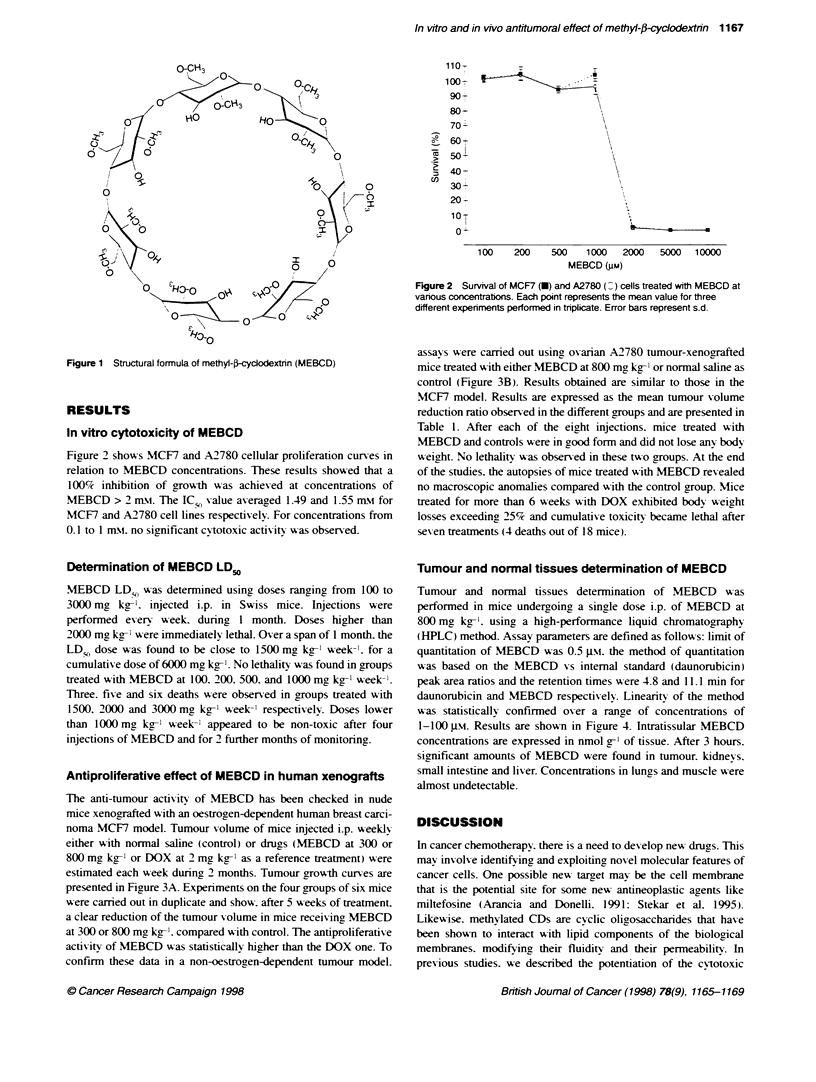
Abstract
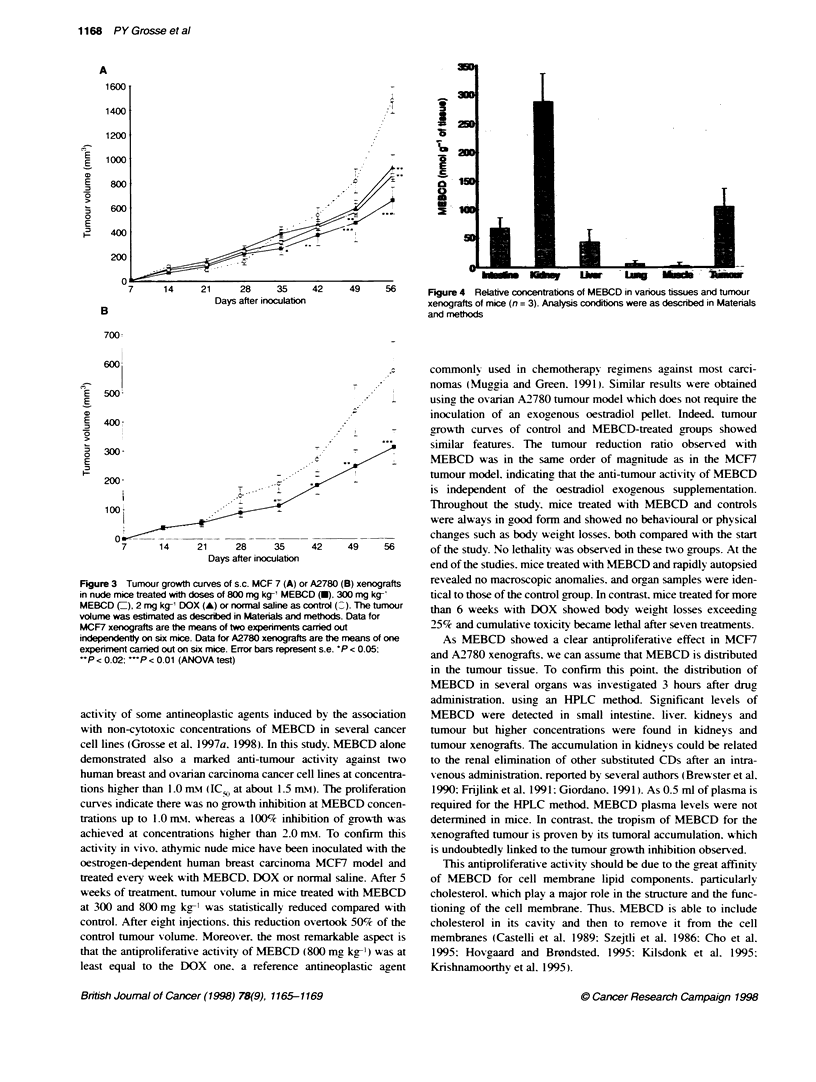
The anti-tumour activity of methyl-beta-cyclodextrin (MEBCD), a cyclic oligosaccharide known for its interaction with the plasma membrane, was investigated in vitro and in vivo and compared with that of doxorubicin (DOX) in the human tumour models MCF7 breast carcinoma and A2780 ovarian carcinoma. In vitro proliferation was assessed using the MTT assay. In vivo studies were carried out using xenografted Swiss nude mice injected weekly i.p. with MEBCD at 300 or 800 mg kg(-1) or DOX at 2 mg kg(-1), during 2 months. Under these conditions, MEBCD was active against MCF7 and A2780 cell lines and tumour xenografts. For each tumour model, the tumoral volume of the xenografted mice treated with MEBCD was at least twofold reduced compared with the control group. In the MCF7 model, MEBCD (800 mg kg(-1)) was more active than DOX (2 mg kg(-1)). After 56 days of treatment with MEBCD, no toxicologically meaningful differences were observed in macroscopic and microscopic parameters compared with controls. The accumulation of MEBCD in normal and tumour tissues was also assessed using a chromatographic method. Results indicated that after a single injection of MEBCD, tumour, liver and kidneys accumulated the highest concentrations of MEBCD. These results provided a basis for the potential therapeutic application of MEBCD in cancer therapy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alley M. C., Scudiero D. A., Monks A., Hursey M. L., Czerwinski M. J., Fine D. L., Abbott B. J., Mayo J. G., Shoemaker R. H., Boyd M. R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988 Feb 1;48(3):589–601. [PubMed] [Google Scholar]
- Arancia G., Donelli G. Cell membranes as target for anticancer agents. Pharmacol Res. 1991 Oct;24(3):205–217. doi: 10.1016/1043-6618(91)90083-a. [DOI] [PubMed] [Google Scholar]
- Bellringer M. E., Smith T. G., Read R., Gopinath C., Olivier P. beta-Cyclodextrin: 52-week toxicity studies in the rat and dog. Food Chem Toxicol. 1995 May;33(5):367–376. doi: 10.1016/0278-6915(94)00149-i. [DOI] [PubMed] [Google Scholar]
- Bressolle F., Audran M., Pham T. N., Vallon J. J. Cyclodextrins and enantiomeric separations of drugs by liquid chromatography and capillary electrophoresis: basic principles and new developments. J Chromatogr B Biomed Appl. 1996 Dec 13;687(2):303–336. doi: 10.1016/s0378-4347(96)00263-0. [DOI] [PubMed] [Google Scholar]
- Cho M. J., Chen F. J., Huczek D. L. Effects of inclusion complexation on the transepithelial transport of a lipophilic substance in vitro. Pharm Res. 1995 Apr;12(4):560–564. doi: 10.1023/a:1016258114283. [DOI] [PubMed] [Google Scholar]
- Colangelo D., Guo H. Y., Connors K. M., Kubota T., Silvestro L., Hoffman R. M. Correlation of drug response in human tumors histocultured in vitro with an image-analysis MTT end point and in vivo xenografted in nude mice. Anticancer Res. 1992 Sep-Oct;12(5):1373–1376. [PubMed] [Google Scholar]
- Flourié B., Molis C., Achour L., Dupas H., Hatat C., Rambaud J. C. Fate of beta-cyclodextrin in the human intestine. J Nutr. 1993 Apr;123(4):676–680. doi: 10.1093/jn/123.4.676. [DOI] [PubMed] [Google Scholar]
- Frijlink H. W., Franssen E. J., Eissens A. C., Oosting R., Lerk C. F., Meijer D. K. The effects of cyclodextrins on the disposition of intravenously injected drugs in the rat. Pharm Res. 1991 Mar;8(3):380–384. doi: 10.1023/a:1015857902238. [DOI] [PubMed] [Google Scholar]
- Frijlink H. W., Visser J., Hefting N. R., Oosting R., Meijer D. K., Lerk C. F. The pharmacokinetics of beta-cyclodextrin and hydroxypropyl-beta-cyclodextrin in the rat. Pharm Res. 1990 Dec;7(12):1248–1252. doi: 10.1023/a:1015929720063. [DOI] [PubMed] [Google Scholar]
- Giordano F. Destino metabolico e profilo tossicologico della idrossipropilbetaciclodestrina. Boll Chim Farm. 1991 Jun;130(6):239–240. [PubMed] [Google Scholar]
- Grosse P. Y., Bressolle F., Pinguet F. In vitro modulation of doxorubicin and docetaxel antitumoral activity by methyl-beta-cyclodextrin. Eur J Cancer. 1998 Jan;34(1):168–174. doi: 10.1016/s0959-8049(97)00351-1. [DOI] [PubMed] [Google Scholar]
- Grosse P. Y., Pinguet F., Joulia J. M., Astre C., Bressolle F. High-performance liquid chromatographic assay for methyl-beta-cyclodextrin in plasma and cell lysate. J Chromatogr B Biomed Sci Appl. 1997 Jun 20;694(1):219–226. doi: 10.1016/s0378-4347(97)00103-5. [DOI] [PubMed] [Google Scholar]
- Heo D. S., Park J. G., Hata K., Day R., Herberman R. B., Whiteside T. L. Evaluation of tetrazolium-based semiautomatic colorimetric assay for measurement of human antitumor cytotoxicity. Cancer Res. 1990 Jun 15;50(12):3681–3690. [PubMed] [Google Scholar]
- Hovgaard L., Brøndsted H. Drug delivery studies in Caco-2 monolayers. IV. Absorption enhancer effects of cyclodextrins. Pharm Res. 1995 Sep;12(9):1328–1332. doi: 10.1023/a:1016225707807. [DOI] [PubMed] [Google Scholar]
- Kilsdonk E. P., Yancey P. G., Stoudt G. W., Bangerter F. W., Johnson W. J., Phillips M. C., Rothblat G. H. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995 Jul 21;270(29):17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- Mimnaugh E. G., Fairchild C. R., Fruehauf J. P., Sinha B. K. Biochemical and pharmacological characterization of MCF-7 drug-sensitive and AdrR multidrug-resistant human breast tumor xenografts in athymic nude mice. Biochem Pharmacol. 1991 Jul 5;42(2):391–402. doi: 10.1016/0006-2952(91)90727-m. [DOI] [PubMed] [Google Scholar]
- Muggia F. M., Green M. D. New anthracycline antitumor antibiotics. Crit Rev Oncol Hematol. 1991;11(1):43–64. doi: 10.1016/1040-8428(91)90017-7. [DOI] [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Stekar J., Hilgard P., Klenner T. Opposite effect of miltefosine on the antineoplastic activity and haematological toxicity of cyclophosphamide. Eur J Cancer. 1995;31A(3):372–374. doi: 10.1016/0959-8049(94)00495-q. [DOI] [PubMed] [Google Scholar]
- Szejtli J. Medicinal applications of cyclodextrins. Med Res Rev. 1994 May;14(3):353–386. doi: 10.1002/med.2610140304. [DOI] [PubMed] [Google Scholar]
- Tomayko M. M., Reynolds C. P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24(3):148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]


