Abstract
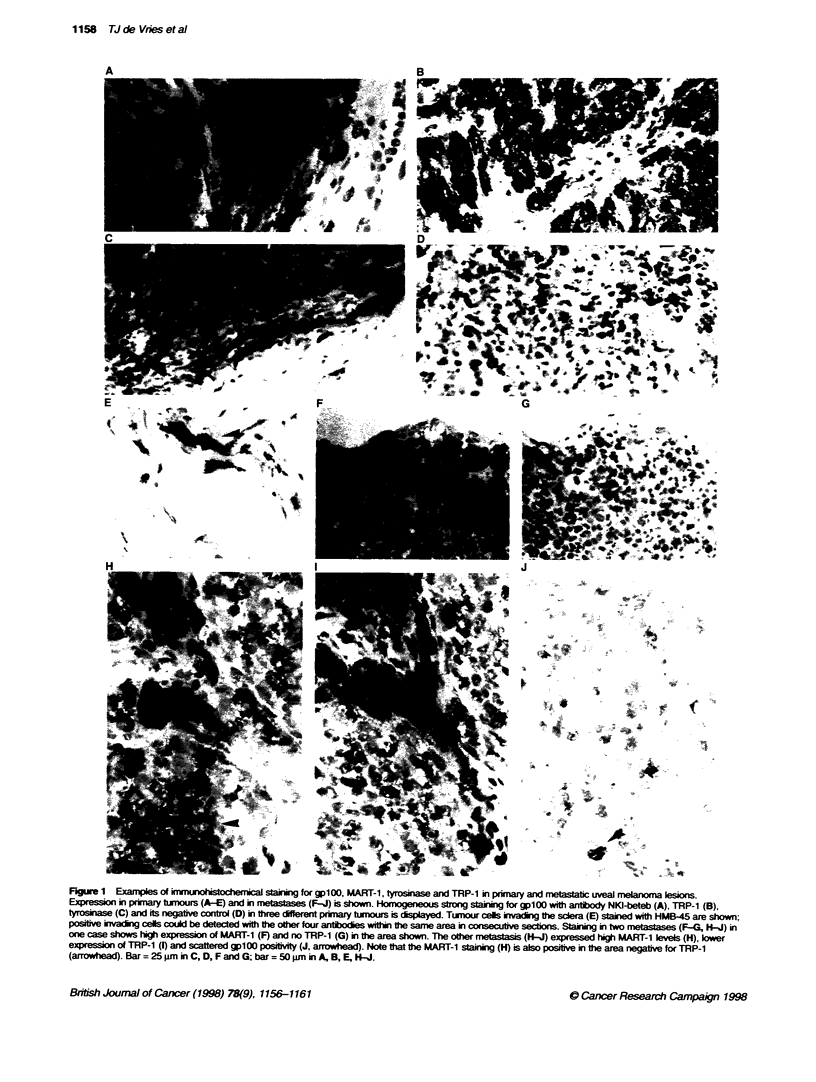
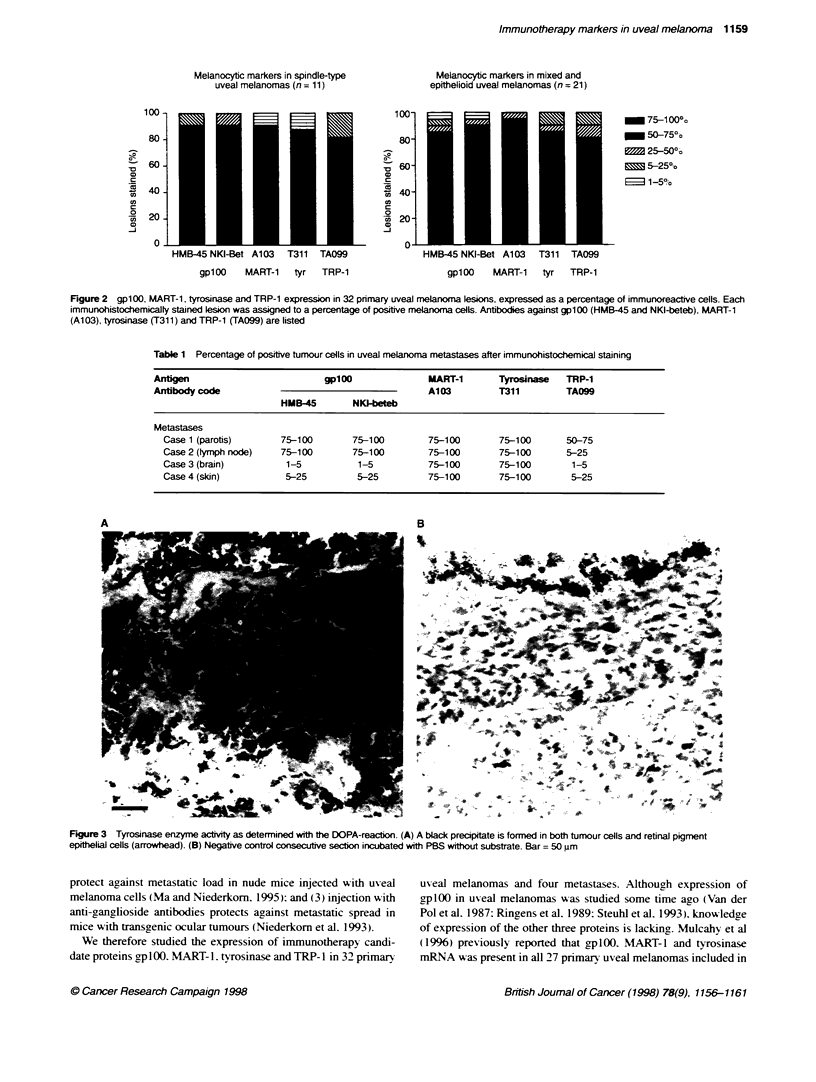
In the treatment of cutaneous melanoma, provisional therapeutic strategies have been designed to combat tumour load using T cells that are sensitized with peptides derived from melanoma autoantigens, such as glycoprotein 100 (gp100), melanoma antigen recognized by T cells 1 (MART-1 or MelanA), tyrosinase and tyrosinase-related protein 1 (TRP-1). We recently found that gp100, MART-1 and tyrosinase are heterogeneously expressed in human cutaneous melanoma (De Vries et al (1997) Cancer Res 57: 3223-3229). Here, we extended our investigations on expression of these immunotherapy candidate proteins to uveal melanoma lesions. Cryostat sections from 11 spindle-type, 21 mixed and epithelioid tumours and four metastasis lesions were stained with antibodies specifically recognizing gp100, MART-1, tyrosinase and TRP-1. In addition, we used the DOPA reaction to detect tyrosinase enzyme activity as a confirmation of the tyrosinase immunohistochemical results. High expression of gp100, MART-1 and tyrosinase was found in the uveal melanoma lesions: 80% of the lesions displayed 75-100% positive tumour cells. TRP-1 positivity was slightly less: approximately 65% of the lesions stained in the 75-100% positive tumour cell category. All uveal melanoma lesions were positive for the four markers studied, this being in contrast to cutaneous melanoma where 17% of the advanced primary lesions and metastases were negative. The presence of these antigens was a little lower in metastases. We conclude that uveal melanomas and their metastases express melanocyte-lineage immunotherapy candidate proteins very abundantly. Uveal melanomas differ in this respect from cutaneous melanoma, in which the expression of these immunotherapy antigens was much more heterogeneous. This makes uveal melanoma a suitable candidate tumour for immunotherapeutic approaches.
Full text
PDF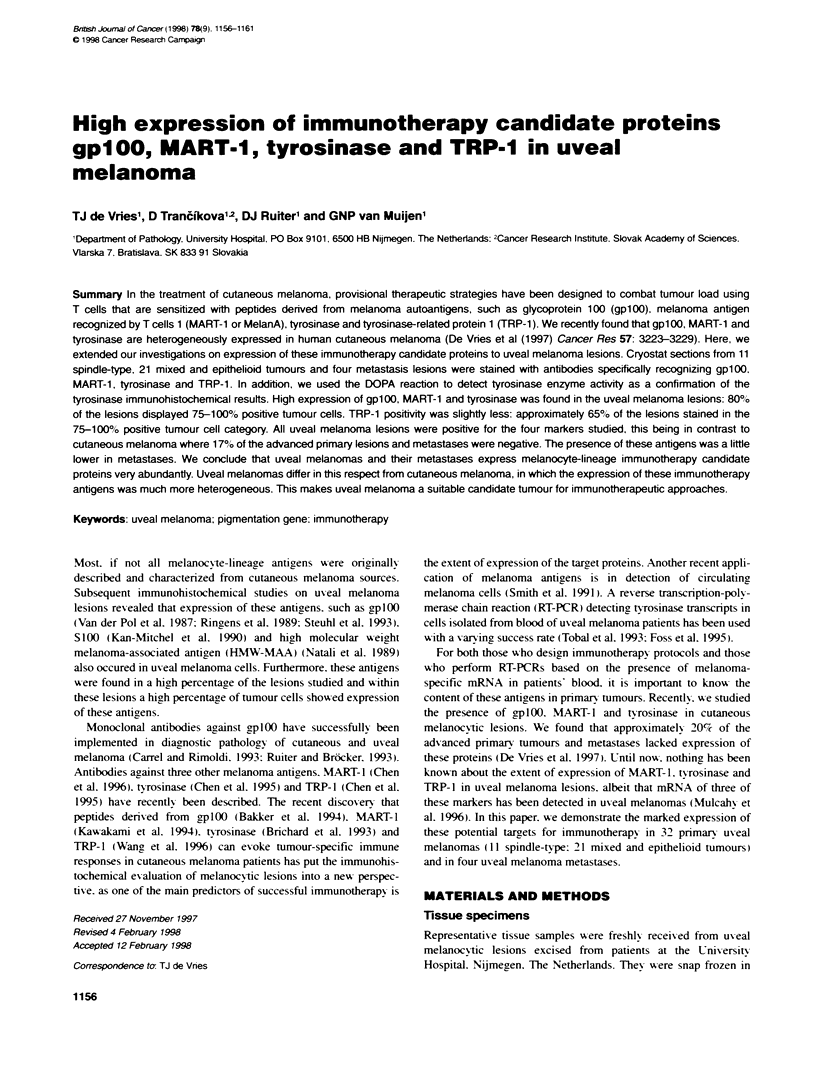



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Durlu Y. K., Tamai M. The properties of retinal pigment epithelial cells in proliferative vitreoretinopathy compared with cultured retinal pigment epithelial cells. Exp Eye Res. 1996 Aug;63(2):201–210. doi: 10.1006/exer.1996.0109. [DOI] [PubMed] [Google Scholar]
- Adema G. J., de Boer A. J., van 't Hullenaar R., Denijn M., Ruiter D. J., Vogel A. M., Figdor C. G. Melanocyte lineage-specific antigens recognized by monoclonal antibodies NKI-beteb, HMB-50, and HMB-45 are encoded by a single cDNA. Am J Pathol. 1993 Dec;143(6):1579–1585. [PMC free article] [PubMed] [Google Scholar]
- Bakker A. B., Schreurs M. W., de Boer A. J., Kawakami Y., Rosenberg S. A., Adema G. J., Figdor C. G. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J Exp Med. 1994 Mar 1;179(3):1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichard V., Van Pel A., Wölfel T., Wölfel C., De Plaen E., Lethé B., Coulie P., Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993 Aug 1;178(2):489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel S., Rimoldi D. Melanoma-associated antigens. Eur J Cancer. 1993;29A(13):1903–1907. doi: 10.1016/0959-8049(93)90548-t. [DOI] [PubMed] [Google Scholar]
- Chan C. C., Detrick B., Nussenblatt R. B., Palestine A. G., Fujikawa L. S., Hooks J. J. HLA-DR antigens on retinal pigment epithelial cells from patients with uveitis. Arch Ophthalmol. 1986 May;104(5):725–729. doi: 10.1001/archopht.1986.01050170115034. [DOI] [PubMed] [Google Scholar]
- Chen Y. T., Stockert E., Jungbluth A., Tsang S., Coplan K. A., Scanlan M. J., Old L. J. Serological analysis of Melan-A(MART-1), a melanocyte-specific protein homogeneously expressed in human melanomas. Proc Natl Acad Sci U S A. 1996 Jun 11;93(12):5915–5919. doi: 10.1073/pnas.93.12.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. T., Stockert E., Tsang S., Coplan K. A., Old L. J. Immunophenotyping of melanomas for tyrosinase: implications for vaccine development. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8125–8129. doi: 10.1073/pnas.92.18.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detrick B., Rodrigues M., Chan C. C., Tso M. O., Hooks J. J. Expression of HLA-DR antigen on retinal pigment epithelial cells in retinitis pigmentosa. Am J Ophthalmol. 1986 May 15;101(5):584–590. doi: 10.1016/0002-9394(86)90949-9. [DOI] [PubMed] [Google Scholar]
- Foss A. J., Guille M. J., Occleston N. L., Hykin P. G., Hungerford J. L., Lightman S. The detection of melanoma cells in peripheral blood by reverse transcription-polymerase chain reaction. Br J Cancer. 1995 Jul;72(1):155–159. doi: 10.1038/bjc.1995.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan-Mitchell J., Liggett P. E., Harel W., Steinman L., Nitta T., Oksenberg J. R., Posner M. R., Mitchell M. S. Lymphocytes cytotoxic to uveal and skin melanoma cells from peripheral blood of ocular melanoma patients. Cancer Immunol Immunother. 1991;33(5):333–340. doi: 10.1007/BF01756599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Sakaguchi K., Robbins P. F., Rivoltini L., Yannelli J. R., Appella E., Rosenberg S. A. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med. 1994 Jul 1;180(1):347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D., Niederkorn J. Y. Efficacy of tumor-infiltrating lymphocytes in the treatment of hepatic metastases arising from transgenic intraocular tumors in mice. Invest Ophthalmol Vis Sci. 1995 May;36(6):1067–1075. [PubMed] [Google Scholar]
- Mulcahy K. A., Rimoldi D., Brasseur F., Rodgers S., Liénard D., Marchand M., Rennie I. G., Murray A. K., McIntyre C. A., Platts K. E. Infrequent expression of the MAGE gene family in uveal melanomas. Int J Cancer. 1996 Jun 11;66(6):738–742. doi: 10.1002/(SICI)1097-0215(19960611)66:6<738::AID-IJC5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Bigotti A., Nicotra M. R., Nardi R. M., Delovu A., Segatto O., Ferrone S. Analysis of the antigenic profile of uveal melanoma lesions with anti-cutaneous melanoma-associated antigen and anti-HLA monoclonal antibodies. Cancer Res. 1989 Mar 1;49(5):1269–1274. [PubMed] [Google Scholar]
- Niederkorn J. Y., Mellon J., Pidherney M., Mayhew E., Anand R. Effect of anti-ganglioside antibodies on the metastatic spread of intraocular melanomas in a nude mouse model of human uveal melanoma. Curr Eye Res. 1993 Apr;12(4):347–358. doi: 10.3109/02713689308999459. [DOI] [PubMed] [Google Scholar]
- Ringens P. J., van Haperen R., Vennegoor C., de Jong P. T., van Duinen S. G., Ruiter D. J., van der Kamp A. W. Monoclonal antibodies in detection of choroidal melanoma. Graefes Arch Clin Exp Ophthalmol. 1989;227(3):287–290. doi: 10.1007/BF02172764. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol Today. 1997 Apr;18(4):175–182. doi: 10.1016/s0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- Ruiter D. J., Bröcker E. B. Immunohistochemistry in the evaluation of melanocytic tumors. Semin Diagn Pathol. 1993 Feb;10(1):76–91. [PubMed] [Google Scholar]
- Smith-Thomas L., Richardson P., Thody A. J., Graham A., Palmer I., Flemming L., Parsons M. A., Rennie I. G., MacNeil S. Human ocular melanocytes and retinal pigment epithelial cells differ in their melanogenic properties in vivo and in vitro. Curr Eye Res. 1996 Nov;15(11):1079–1091. doi: 10.3109/02713689608995139. [DOI] [PubMed] [Google Scholar]
- Smith B., Selby P., Southgate J., Pittman K., Bradley C., Blair G. E. Detection of melanoma cells in peripheral blood by means of reverse transcriptase and polymerase chain reaction. Lancet. 1991 Nov 16;338(8777):1227–1229. doi: 10.1016/0140-6736(91)92100-g. [DOI] [PubMed] [Google Scholar]
- Steuhl K. P., Rohrbach J. M., Knorr M., Thiel H. J. Significance, specificity, and ultrastructural localization of HMB-45 antigen in pigmented ocular tumors. Ophthalmology. 1993 Feb;100(2):208–215. doi: 10.1016/s0161-6420(93)31668-4. [DOI] [PubMed] [Google Scholar]
- Sugita S., Sagawa K., Mochizuki M., Shichijo S., Itoh K. Melanocyte lysis by cytotoxic T lymphocytes recognizing the MART-1 melanoma antigen in HLA-A2 patients with Vogt-Koyanagi-Harada disease. Int Immunol. 1996 May;8(5):799–803. doi: 10.1093/intimm/8.5.799. [DOI] [PubMed] [Google Scholar]
- Tobal K., Sherman L. S., Foss A. J., Lightman S. L. Detection of melanocytes from uveal melanoma in peripheral blood using the polymerase chain reaction. Invest Ophthalmol Vis Sci. 1993 Aug;34(9):2622–2625. [PubMed] [Google Scholar]
- Wang R. F., Parkhurst M. R., Kawakami Y., Robbins P. F., Rosenberg S. A. Utilization of an alternative open reading frame of a normal gene in generating a novel human cancer antigen. J Exp Med. 1996 Mar 1;183(3):1131–1140. doi: 10.1084/jem.183.3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries T. J., Fourkour A., Wobbes T., Verkroost G., Ruiter D. J., van Muijen G. N. Heterogeneous expression of immunotherapy candidate proteins gp100, MART-1, and tyrosinase in human melanoma cell lines and in human melanocytic lesions. Cancer Res. 1997 Aug 1;57(15):3223–3229. [PubMed] [Google Scholar]
- de Vries T. J., Verheijen J. H., de Bart A. C., Weidle U. H., Ruiter D. J., van Muijen G. N. Decreased expression of both the low-density lipoprotein receptor-related protein/alpha(2)-macroglobulin receptor and its receptor-associated protein in late stages of cutaneous melanocytic tumor progression. Cancer Res. 1996 Mar 15;56(6):1432–1439. [PubMed] [Google Scholar]
- van der Pol J. P., Jager M. J., de Wolff-Rouendaal D., Ringens P. J., Vennegoor C., Ruiter D. J. Heterogeneous expression of melanoma-associated antigens in uveal melanomas. Curr Eye Res. 1987 Jun;6(6):757–765. doi: 10.3109/02713688709034842. [DOI] [PubMed] [Google Scholar]