Abstract
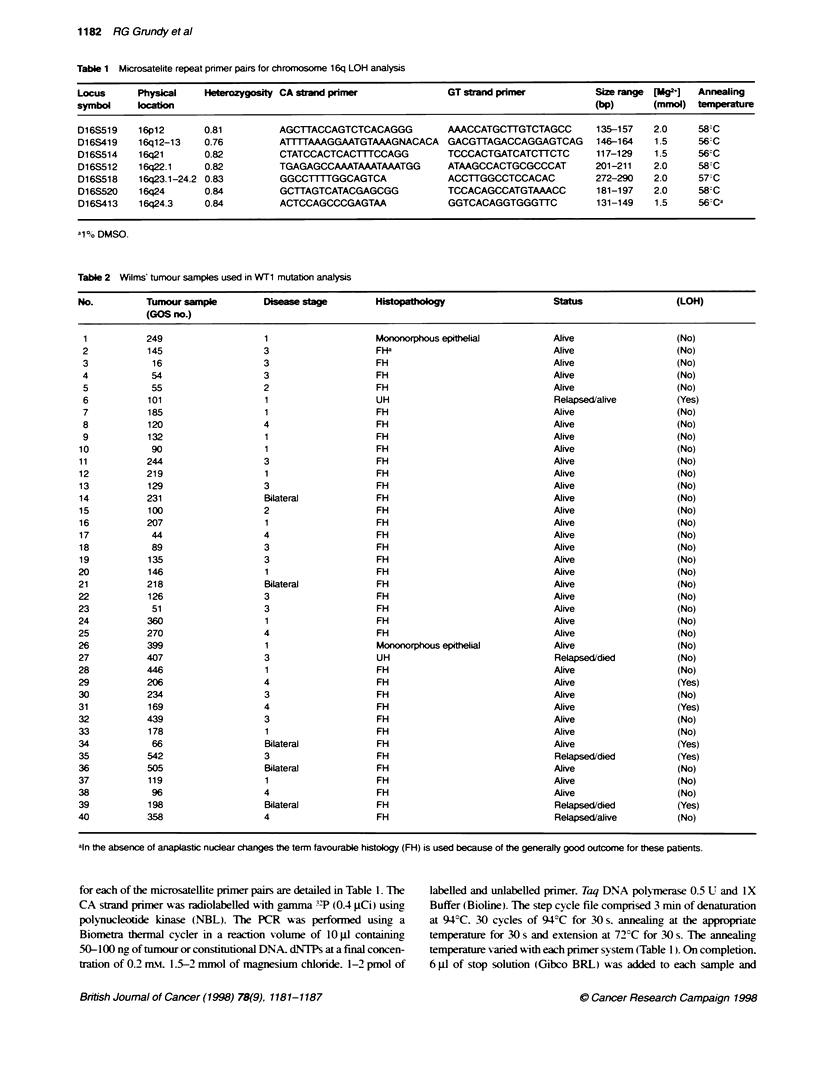
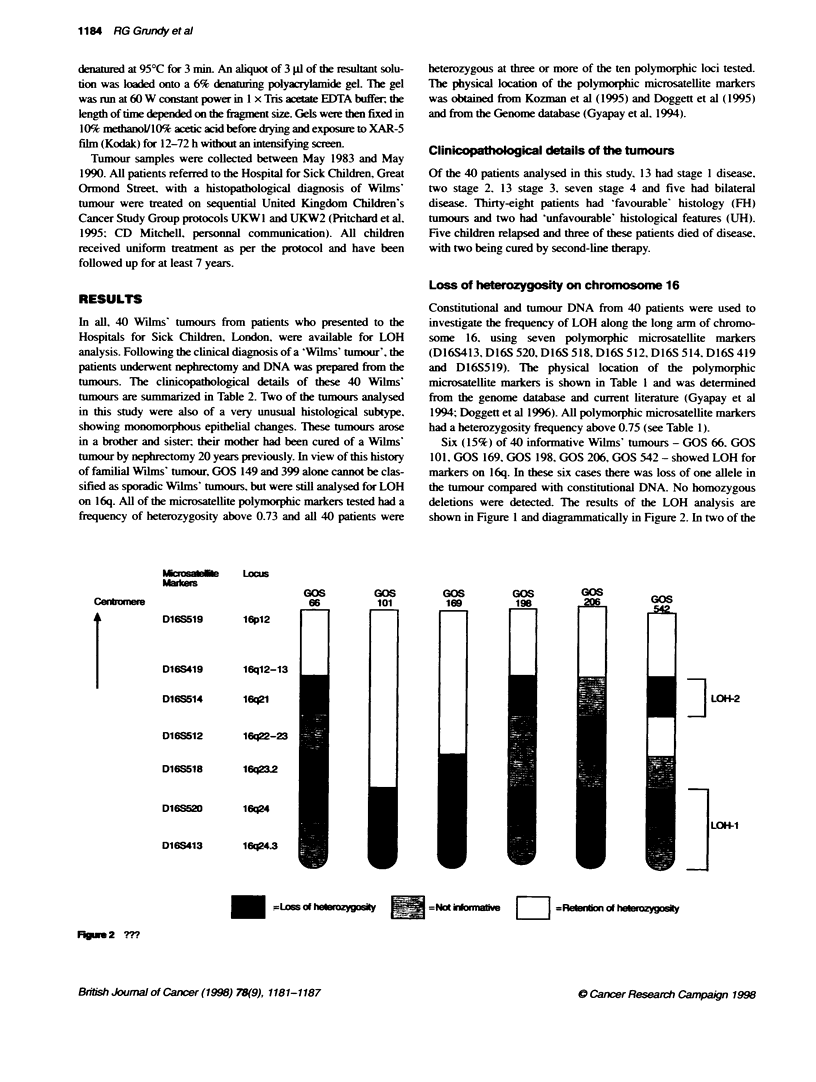
To establish whether loss of heterozygosity (LOH) for chromosome 16q in Wilms' tumours confers an adverse prognosis, DNA from 40 Wilms' tumour/normal pairs were analysed using highly polymorphic microsatellite markers along the length of 16q. Fifteen per cent of tumours showed LOH for 16q. Although the common region of allele loss spanned the 16q24-qter region, a second distinct region of LOH was identified in 16q21. Five out of six tumours showing LOH were either (1) high stage or (2) low stage with unfavourable histology. In addition, there was a higher mortality rate in patients showing LOH for 16q than those that did not. These data strongly support the suggestion that LOH for 16q is associated with an adverse prognosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austruy E., Candon S., Henry I., Gyapay G., Tournade M. F., Mannens M., Callen D., Junien C., Jeanpierre C. Characterization of regions of chromosomes 12 and 16 involved in nephroblastoma tumorigenesis. Genes Chromosomes Cancer. 1995 Dec;14(4):285–294. doi: 10.1002/gcc.2870140407. [DOI] [PubMed] [Google Scholar]
- Beckwith J. B., Kiviat N. B., Bonadio J. F. Nephrogenic rests, nephroblastomatosis, and the pathogenesis of Wilms' tumor. Pediatr Pathol. 1990;10(1-2):1–36. doi: 10.3109/15513819009067094. [DOI] [PubMed] [Google Scholar]
- Bergerheim U. S., Kunimi K., Collins V. P., Ekman P. Deletion mapping of chromosomes 8, 10, and 16 in human prostatic carcinoma. Genes Chromosomes Cancer. 1991 May;3(3):215–220. doi: 10.1002/gcc.2870030308. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- D'Angio G. J., Evans A., Breslow N., Beckwith B., Bishop H., Farewell V., Goodwin W., Leape L., Palmer N., Sinks L. The treatment of Wilms' tumor: results of the Second National Wilms' Tumor Study. Cancer. 1981 May 1;47(9):2302–2311. doi: 10.1002/1097-0142(19810501)47:9<2302::aid-cncr2820470933>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Dorion-Bonnet F., Mautalen S., Hostein I., Longy M. Allelic imbalance study of 16q in human primary breast carcinomas using microsatellite markers. Genes Chromosomes Cancer. 1995 Nov;14(3):171–181. doi: 10.1002/gcc.2870140304. [DOI] [PubMed] [Google Scholar]
- Douglass E. C., Rowe S. T., Valentine M., Parham D., Meyer W. H., Thompson E. I. A second nonrandom translocation, der(16)t(1;16)(q21;q13), in Ewing sarcoma and peripheral neuroectodermal tumor. Cytogenet Cell Genet. 1990;53(2-3):87–90. doi: 10.1159/000132901. [DOI] [PubMed] [Google Scholar]
- Fujimori M., Tokino T., Hino O., Kitagawa T., Imamura T., Okamoto E., Mitsunobu M., Ishikawa T., Nakagama H., Harada H. Allelotype study of primary hepatocellular carcinoma. Cancer Res. 1991 Jan 1;51(1):89–93. [PubMed] [Google Scholar]
- Goorin A. M., Chauvenet A. R., Perez-Atayde A. R., Cruz J., McKone R., Lipshultz S. E. Initial congestive heart failure, six to ten years after doxorubicin chemotherapy for childhood cancer. J Pediatr. 1990 Jan;116(1):144–147. doi: 10.1016/s0022-3476(05)81668-3. [DOI] [PubMed] [Google Scholar]
- Grundy P. E., Telzerow P. E., Breslow N., Moksness J., Huff V., Paterson M. C. Loss of heterozygosity for chromosomes 16q and 1p in Wilms' tumors predicts an adverse outcome. Cancer Res. 1994 May 1;54(9):2331–2333. [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Kaneko Y., Homma C., Maseki N., Sakurai M., Hata J. Correlation of chromosome abnormalities with histological and clinical features in Wilms' and other childhood renal tumors. Cancer Res. 1991 Nov 1;51(21):5937–5942. [PubMed] [Google Scholar]
- Knudson A. G., Jr, Strong L. C. Mutation and cancer: a model for Wilms' tumor of the kidney. J Natl Cancer Inst. 1972 Feb;48(2):313–324. [PubMed] [Google Scholar]
- Koufos A., Grundy P., Morgan K., Aleck K. A., Hadro T., Lampkin B. C., Kalbakji A., Cavenee W. K. Familial Wiedemann-Beckwith syndrome and a second Wilms tumor locus both map to 11p15.5. Am J Hum Genet. 1989 May;44(5):711–719. [PMC free article] [PubMed] [Google Scholar]
- Kozman H. M., Keith T. P., Donis-Keller H., White R. L., Weissenbach J., Dean M., Vergnaud G., Kidd K., Gusella J., Royle N. J. The CEPH consortium linkage map of human chromosome 16. Genomics. 1995 Jan 1;25(1):44–58. doi: 10.1016/0888-7543(95)80108-x. [DOI] [PubMed] [Google Scholar]
- Mugneret F., Lizard S., Aurias A., Turc-Carel C. Chromosomes in Ewing's sarcoma. II. Nonrandom additional changes, trisomy 8 and der(16)t(1;16). Cancer Genet Cytogenet. 1988 Jun;32(2):239–245. doi: 10.1016/0165-4608(88)90286-5. [DOI] [PubMed] [Google Scholar]
- Park S., Bernard A., Bove K. E., Sens D. A., Hazen-Martin D. J., Garvin A. J., Haber D. A. Inactivation of WT1 in nephrogenic rests, genetic precursors to Wilms' tumour. Nat Genet. 1993 Dec;5(4):363–367. doi: 10.1038/ng1293-363. [DOI] [PubMed] [Google Scholar]
- Ping A. J., Reeve A. E., Law D. J., Young M. R., Boehnke M., Feinberg A. P. Genetic linkage of Beckwith-Wiedemann syndrome to 11p15. Am J Hum Genet. 1989 May;44(5):720–723. [PMC free article] [PubMed] [Google Scholar]
- Pritchard J., Imeson J., Barnes J., Cotterill S., Gough D., Marsden H. B., Morris-Jones P., Pearson D. Results of the United Kingdom Children's Cancer Study Group first Wilms' Tumor Study. J Clin Oncol. 1995 Jan;13(1):124–133. doi: 10.1200/JCO.1995.13.1.124. [DOI] [PubMed] [Google Scholar]
- Sato T., Tanigami A., Yamakawa K., Akiyama F., Kasumi F., Sakamoto G., Nakamura Y. Allelotype of breast cancer: cumulative allele losses promote tumor progression in primary breast cancer. Cancer Res. 1990 Nov 15;50(22):7184–7189. [PubMed] [Google Scholar]
- Sheng W. W., Soukup S., Bove K., Gotwals B., Lampkin B. Chromosome analysis of 31 Wilms' tumors. Cancer Res. 1990 May 1;50(9):2786–2793. [PubMed] [Google Scholar]
- Slater R. M., Mannens M. M. Cytogenetics and molecular genetics of Wilms' tumor of childhood. Cancer Genet Cytogenet. 1992 Jul 15;61(2):111–121. doi: 10.1016/0165-4608(92)90071-f. [DOI] [PubMed] [Google Scholar]
- Solis V., Pritchard J., Cowell J. K. Cytogenetic changes in Wilms' tumors. Cancer Genet Cytogenet. 1988 Sep;34(2):223–234. doi: 10.1016/0165-4608(88)90264-6. [DOI] [PubMed] [Google Scholar]
- Sorensen K., Levitt G., Sebag-Montefiore D., Bull C., Sullivan I. Cardiac function in Wilms' tumor survivors. J Clin Oncol. 1995 Jul;13(7):1546–1556. doi: 10.1200/JCO.1995.13.7.1546. [DOI] [PubMed] [Google Scholar]
- Thomas G. A., Raffel C. Loss of heterozygosity on 6q, 16q, and 17p in human central nervous system primitive neuroectodermal tumors. Cancer Res. 1991 Jan 15;51(2):639–643. [PubMed] [Google Scholar]
- Tsuda H., Zhang W. D., Shimosato Y., Yokota J., Terada M., Sugimura T., Miyamura T., Hirohashi S. Allele loss on chromosome 16 associated with progression of human hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6791–6794. doi: 10.1073/pnas.87.17.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadey R. B., Pal N., Buckle B., Yeomans E., Pritchard J., Cowell J. K. Loss of heterozygosity in Wilms' tumour involves two distinct regions of chromosome 11. Oncogene. 1990 Jun;5(6):901–907. [PubMed] [Google Scholar]
- Weksberg R., Teshima I., Williams B. R., Greenberg C. R., Pueschel S. M., Chernos J. E., Fowlow S. B., Hoyme E., Anderson I. J., Whiteman D. A. Molecular characterization of cytogenetic alterations associated with the Beckwith-Wiedemann syndrome (BWS) phenotype refines the localization and suggests the gene for BWS is imprinted. Hum Mol Genet. 1993 May;2(5):549–556. doi: 10.1093/hmg/2.5.549. [DOI] [PubMed] [Google Scholar]



