Abstract
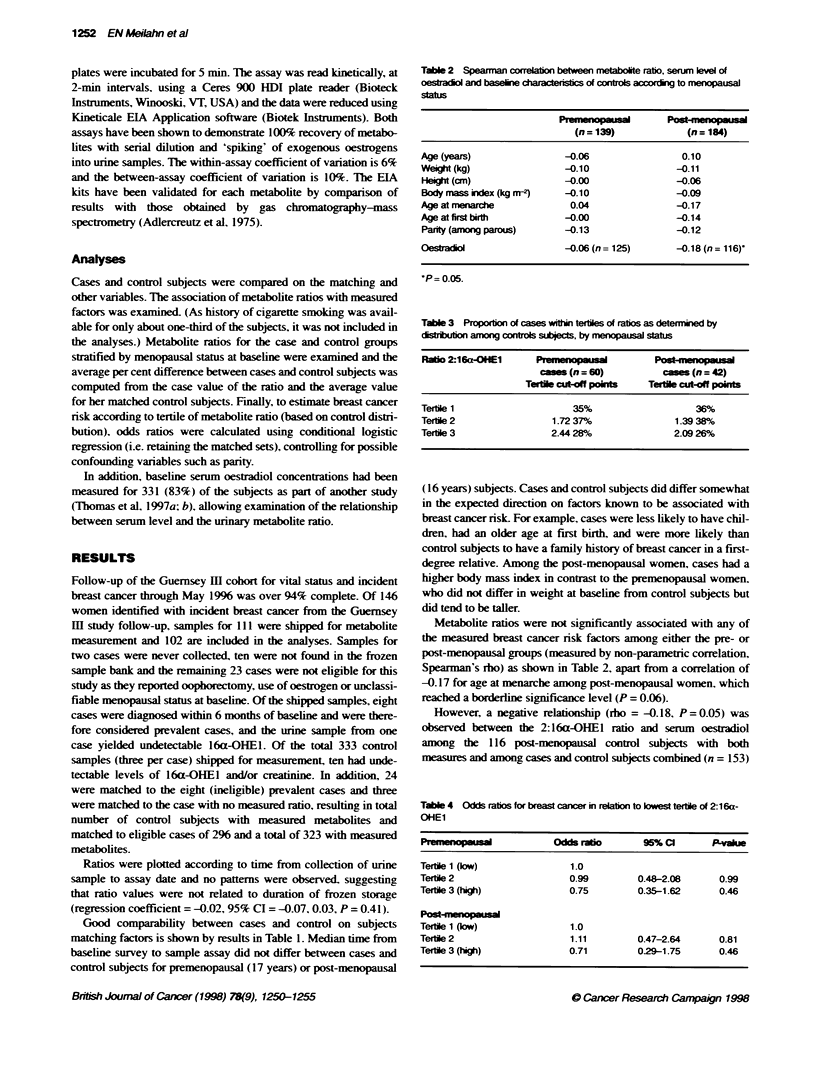
This is the first prospective study of urinary measures of the two major competing pathways of oestrogen metabolism, 16alpha-hydroxyoestrone (16alpha-OHE1) and 2-hydroxyoestrone (2-OHE1), in relation to incident breast cancer risk. Experimental and case-control study results suggest that metabolism favouring the more oestrogenic 16alpha-OHE1 pathway may be linked to higher breast cancer risk. Women aged 35 and older from Guernsey (n = 5104) were surveyed in 1977-85 and have been continuously monitored for breast cancer and mortality up to the present (Guernsey III, Imperial Cancer Research Fund). Incident cases of breast cancer were matched to three control subjects for comparison of urinary oestrogen metabolite levels measured by enzyme immunoassay (EIA) in spot urine samples collected at baseline and stored frozen for up to 19 years. Consistent with case-control study results, post-menopausal (but not premenopausal) women at baseline who went on to develop breast cancer showed about a 15% lower 2:16alpha-OHE1 ratio than matched control subjects. Further, subjects with metabolite ratios in the highest tertile of 2:16alpha-OHE1 had about a 30% lower risk than women with ratios in the lowest two-thirds, although results were not statistically significant (OR = 0.71, 95% CI = 0.29-1.75). It is of potential importance that, in contrast to most risk factors for breast cancer, such as late age at first birth, oestrogen metabolism appears to be modifiable via diet and exercise, offering women the possibility of lowering breast cancer risk through non-pharmacological measures, although this remains to be tested.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlercreutz H., Fotsis T., Höckerstedt K., Hämäläinen E., Bannwart C., Bloigu S., Valtonen A., Ollus A. Diet and urinary estrogen profile in premenopausal omnivorous and vegetarian women and in premenopausal women with breast cancer. J Steroid Biochem. 1989;34(1-6):527–530. doi: 10.1016/0022-4731(89)90138-6. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H., Martin F., Wahlroos O., Soini E. Mass spectrometric and mass fragmentographic determination of natural and synthetic steroids in biological fluids. J Steroid Biochem. 1975 Mar-Apr;6(3-4):247–259. doi: 10.1016/0022-4731(75)90140-5. [DOI] [PubMed] [Google Scholar]
- Baron J. A., Newcomb P. A., Longnecker M. P., Mittendorf R., Storer B. E., Clapp R. W., Bogdan G., Yuen J. Cigarette smoking and breast cancer. Cancer Epidemiol Biomarkers Prev. 1996 May;5(5):399–403. [PubMed] [Google Scholar]
- Bradlow H. L., Hershcopf R. J., Martucci C. P., Fishman J. Estradiol 16 alpha-hydroxylation in the mouse correlates with mammary tumor incidence and presence of murine mammary tumor virus: a possible model for the hormonal etiology of breast cancer in humans. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6295–6299. doi: 10.1073/pnas.82.18.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradlow H. L., Michnovicz J., Telang N. T., Osborne M. P. Effects of dietary indole-3-carbinol on estradiol metabolism and spontaneous mammary tumors in mice. Carcinogenesis. 1991 Sep;12(9):1571–1574. doi: 10.1093/carcin/12.9.1571. [DOI] [PubMed] [Google Scholar]
- Fishman J., Martucci C. Biological properties of 16 alpha-hydroxyestrone: implications in estrogen physiology and pathophysiology. J Clin Endocrinol Metab. 1980 Sep;51(3):611–615. doi: 10.1210/jcem-51-3-611. [DOI] [PubMed] [Google Scholar]
- Kabat G. C., Chang C. J., Sparano J. A., Sepkovie D. W., Hu X. P., Khalil A., Rosenblatt R., Bradlow H. L. Urinary estrogen metabolites and breast cancer: a case-control study. Cancer Epidemiol Biomarkers Prev. 1997 Jul;6(7):505–509. [PubMed] [Google Scholar]
- Klug T. L., Bradlow H. L., Sepkovic D. W. Monoclonal antibody-based enzyme immunoassay for simultaneous quantitation of 2- and 16 alpha-hydroxyestrone in urine. Steroids. 1994 Nov;59(11):648–655. doi: 10.1016/0039-128x(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Lemon H. M., Heidel J. W., Rodriguez-Sierra J. F. Increased catechol estrogen metabolism as a risk factor for nonfamilial breast cancer. Cancer. 1992 Jan 15;69(2):457–465. doi: 10.1002/1097-0142(19920115)69:2<457::aid-cncr2820690231>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Longcope C., Gorbach S., Goldin B., Woods M., Dwyer J., Morrill A., Warram J. The effect of a low fat diet on estrogen metabolism. J Clin Endocrinol Metab. 1987 Jun;64(6):1246–1250. doi: 10.1210/jcem-64-6-1246. [DOI] [PubMed] [Google Scholar]
- Lottering M. L., Haag M., Seegers J. C. Effects of 17 beta-estradiol metabolites on cell cycle events in MCF-7 cells. Cancer Res. 1992 Nov 1;52(21):5926–5932. [PubMed] [Google Scholar]
- Martucci C. P., Fishman J. Impact of continuously administered catechol estrogens on uterine growth and luteinizing hormone secretion. Endocrinology. 1979 Dec;105(6):1288–1292. doi: 10.1210/endo-105-6-1288. [DOI] [PubMed] [Google Scholar]
- Michnovicz J. J., Adlercreutz H., Bradlow H. L. Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J Natl Cancer Inst. 1997 May 21;89(10):718–723. doi: 10.1093/jnci/89.10.718. [DOI] [PubMed] [Google Scholar]
- Michnovicz J. J., Hershcopf R. J., Naganuma H., Bradlow H. L., Fishman J. Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med. 1986 Nov 20;315(21):1305–1309. doi: 10.1056/NEJM198611203152101. [DOI] [PubMed] [Google Scholar]
- Pasagian-Macaulay A., Meilahn E. N., Bradlow H. L., Sepkovic D. W., Buhari A. M., Simkin-Silverman L., Wing R. R., Kuller L. H. Urinary markers of estrogen metabolism 2- and 16 alpha-hydroxylation in premenopausal women. Steroids. 1996 Aug;61(8):461–467. doi: 10.1016/0039-128x(96)00089-x. [DOI] [PubMed] [Google Scholar]
- Schneider J., Kinne D., Fracchia A., Pierce V., Anderson K. E., Bradlow H. L., Fishman J. Abnormal oxidative metabolism of estradiol in women with breast cancer. Proc Natl Acad Sci U S A. 1982 May;79(9):3047–3051. doi: 10.1073/pnas.79.9.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow R. C., Barbieri R. L., Frisch R. E. Estrogen 2-hydroxylase oxidation and menstrual function among elite oarswomen. J Clin Endocrinol Metab. 1989 Aug;69(2):369–376. doi: 10.1210/jcem-69-2-369. [DOI] [PubMed] [Google Scholar]
- Telang N. T., Katdare M., Bradlow H. L., Osborne M. P. Estradiol metabolism: an endocrine biomarker for modulation of human mammary carcinogenesis. Environ Health Perspect. 1997 Apr;105 (Suppl 3):559–564. doi: 10.1289/ehp.97105s3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telang N. T. Oncogenes, estradiol biotransformation, and mammary carcinogenesis. Ann N Y Acad Sci. 1996 Apr 30;784:277–287. doi: 10.1111/j.1749-6632.1996.tb16242.x. [DOI] [PubMed] [Google Scholar]
- Telang N. T., Suto A., Wong G. Y., Osborne M. P., Bradlow H. L. Induction by estrogen metabolite 16 alpha-hydroxyestrone of genotoxic damage and aberrant proliferation in mouse mammary epithelial cells. J Natl Cancer Inst. 1992 Apr 15;84(8):634–638. doi: 10.1093/jnci/84.8.634. [DOI] [PubMed] [Google Scholar]
- Thomas H. V., Key T. J., Allen D. S., Moore J. W., Dowsett M., Fentiman I. S., Wang D. Y. A prospective study of endogenous serum hormone concentrations and breast cancer risk in post-menopausal women on the island of Guernsey. Br J Cancer. 1997;76(3):401–405. doi: 10.1038/bjc.1997.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H. V., Key T. J., Allen D. S., Moore J. W., Dowsett M., Fentiman I. S., Wang D. Y. A prospective study of endogenous serum hormone concentrations and breast cancer risk in premenopausal women on the island of Guernsey. Br J Cancer. 1997;75(7):1075–1079. doi: 10.1038/bjc.1997.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin G., London S., Stanczyk F. Z., Gentzschein E., Paganini-Hill A., Ross R. K., Pike M. C. A pilot study of urinary estrogen metabolites (16alpha-OHE1 and 2-OHE1) in postmenopausal women with and without breast cancer. Environ Health Perspect. 1997 Apr;105 (Suppl 3):601–605. doi: 10.1289/ehp.97105s3601. [DOI] [PMC free article] [PubMed] [Google Scholar]


