Abstract
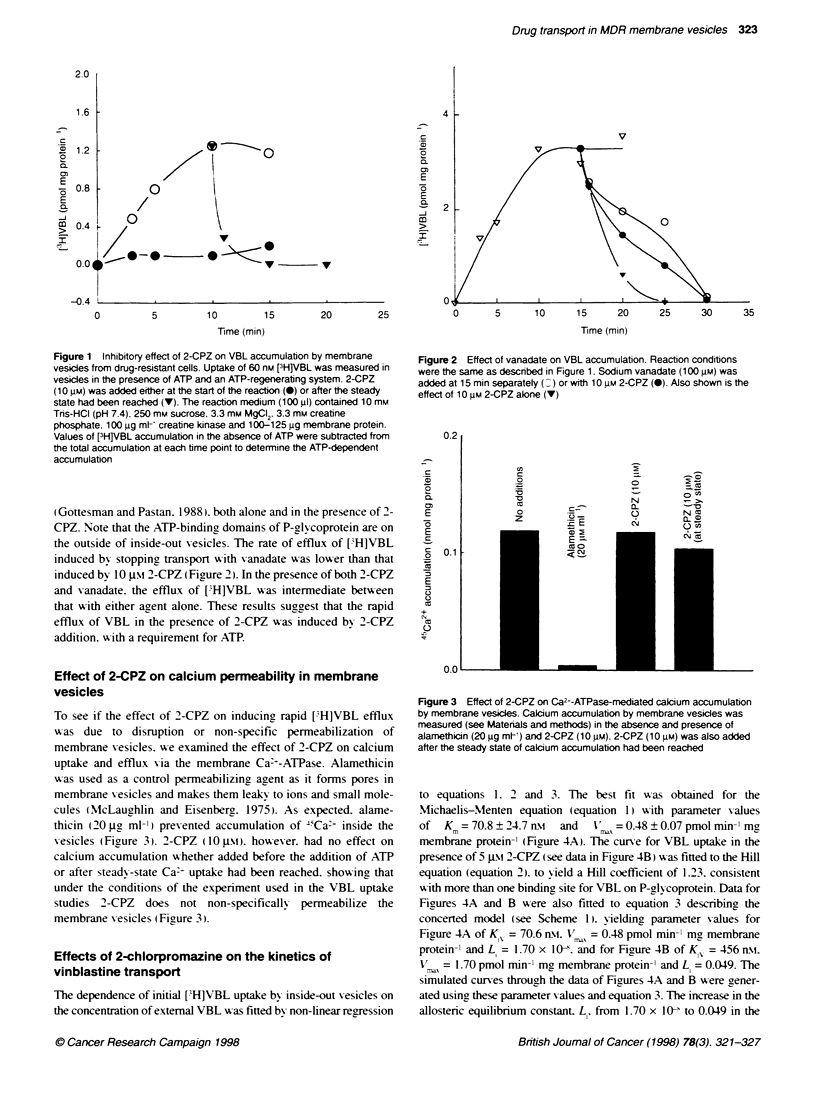
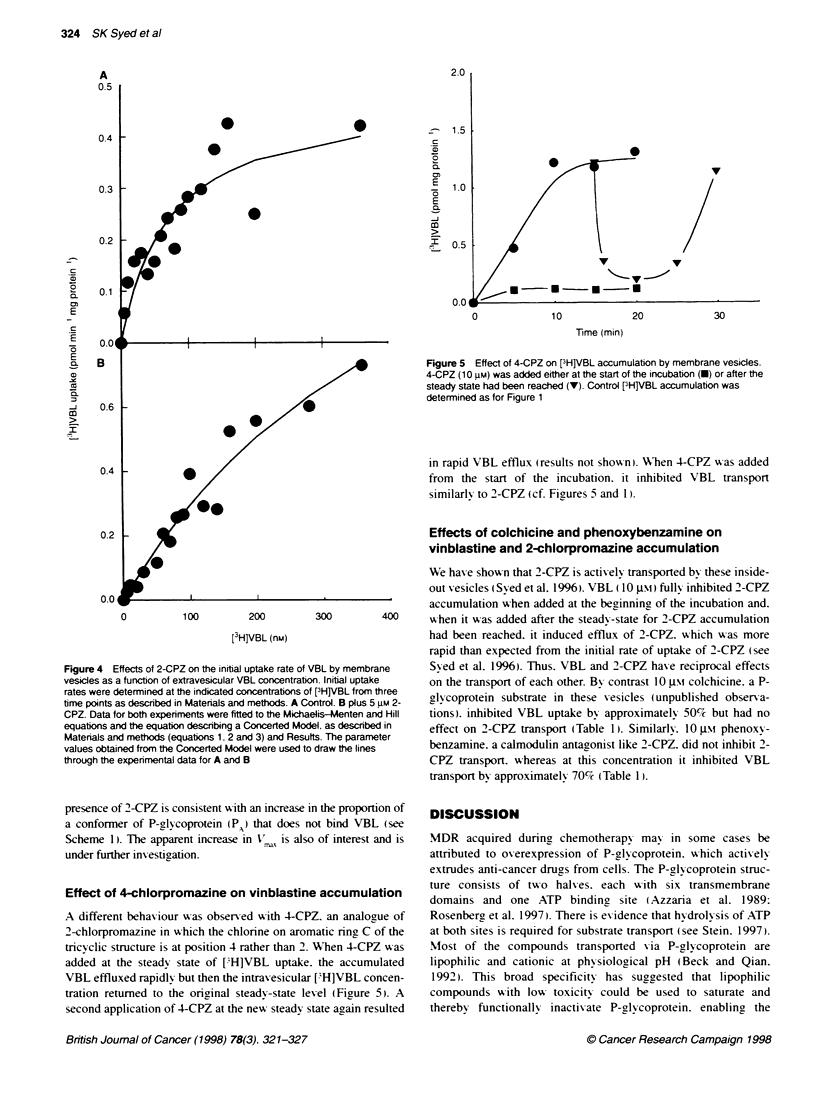
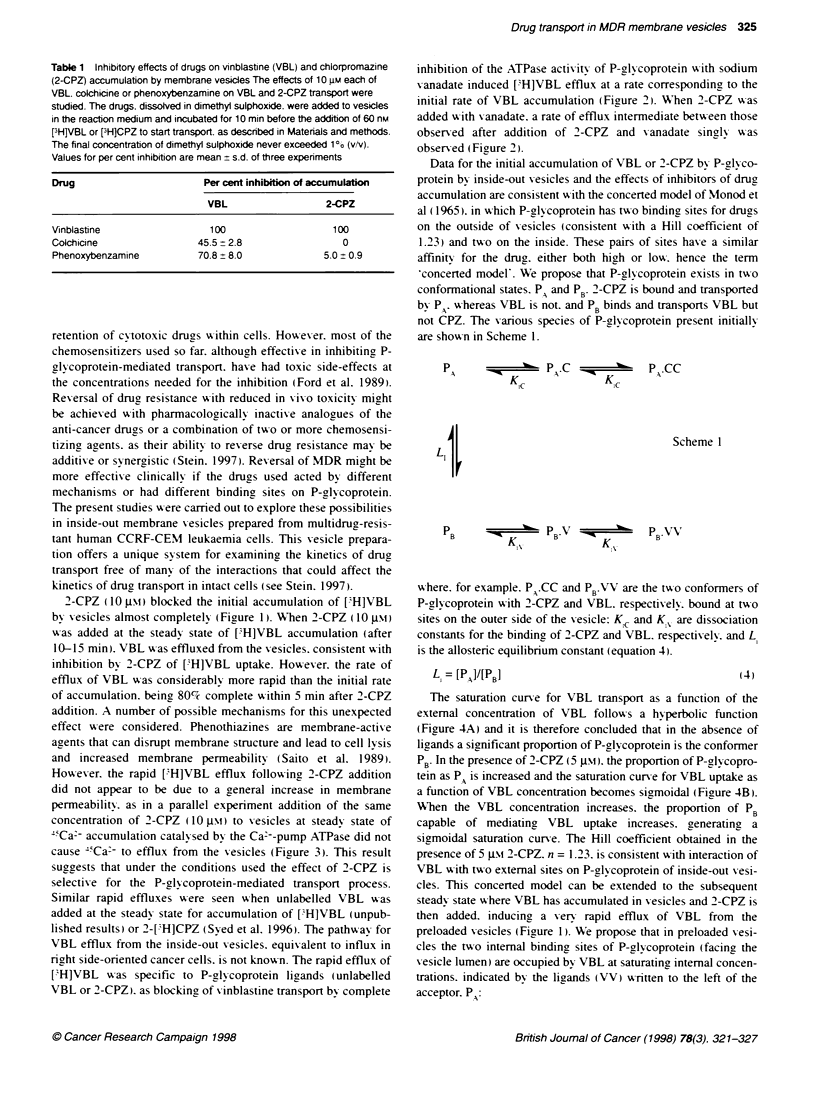
The mechanism of action of 2-chlorpromazine (2-chloro-10-(3-dimethylaminopropyl)-phenothiazine) as a reversal agent for P-glycoprotein-mediated multidrug resistance was investigated using inside out-orientated membrane vesicles prepared from vinblastine-resistant human CCRF-CEM leukaemia cells (VBL1000). 2-Chlorpromazine (10 microM) completely inhibited ATP-dependent P-glycoprotein-mediated vinblastine accumulation in the vesicles. Whereas in the absence of added ligands VBL transport was described by a hyperbolic function of vinblastine concentration, in the presence of 2-chlorpromazine vinblastine transport was a sigmoidal function. 2-Chlorpromazine was shown previously [Syed SK, Christopherson RI and Roufogalis BD (1996) Biochem Mol Biol Int 39: 687-696] to be actively transported into vesicles from multidrug-resistant cells. Colchicine (10 microM) and phenoxybenzamine (10 microM) blocked vinblastine transport but had no effect on 2-chlorpromazine transport into vesicles. The results were consistent with a two-state concerted model in which P-glycoprotein exists in two conformational states, P(A) and P(B), where 2-chlorpromazine is transported by the conformer, P(A), and vinblastine by the conformer, P(B). In the presence of 2-chlorpromazine, the conformer P(A) predominates and vinblastine transport is inhibited. Addition of 2-chlorpromazine during the steady state of vinblastine accumulation blocked uptake and resulted in enhanced vinblastine efflux from the vesicles. The findings were similar when vinblastine was added at the steady state of 2-chlorpromazine transport. We propose a minimal kinetic model whereby in these preloaded vesicles the complex VV.P(A).CC is formed, where two internal binding sites of P-glycoprotein (P(A)) are occupied by vinblastine (V) and the two external sites are occupied by 2-chlorpromazine (C). When the two binding sites on both the inside and outside of P-glycoprotein are saturated with ligands vinblastine is effluxed at a very rapid rate, and vice versa when vesicles are preloaded with 2-chlorpromazine and vinblastine is added outside. These unexpected observations and the concerted model developed provide an alternative mechanism of action for reversal agents that sensitize multidrug-resistant cancer cells to anti-cancer drugs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S., Cornwell M. M., Kuwano M., Pastan I., Gottesman M. M. Most drugs that reverse multidrug resistance also inhibit photoaffinity labeling of P-glycoprotein by a vinblastine analog. Mol Pharmacol. 1988 Feb;33(2):144–147. [PubMed] [Google Scholar]
- Arias I. M. Multidrug resistance genes, p-glycoprotein and the liver. Hepatology. 1990 Jul;12(1):159–165. doi: 10.1002/hep.1840120125. [DOI] [PubMed] [Google Scholar]
- Azzaria M., Schurr E., Gros P. Discrete mutations introduced in the predicted nucleotide-binding sites of the mdr1 gene abolish its ability to confer multidrug resistance. Mol Cell Biol. 1989 Dec;9(12):5289–5297. doi: 10.1128/mcb.9.12.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck W. T., Qian X. D. Photoaffinity substrates for P-glycoprotein. Biochem Pharmacol. 1992 Jan 9;43(1):89–93. doi: 10.1016/0006-2952(92)90665-6. [DOI] [PubMed] [Google Scholar]
- Ferry D. R., Russell M. A., Cullen M. H. P-glycoprotein possesses a 1,4-dihydropyridine-selective drug acceptor site which is alloserically coupled to a vinca-alkaloid-selective binding site. Biochem Biophys Res Commun. 1992 Oct 15;188(1):440–445. doi: 10.1016/0006-291x(92)92404-l. [DOI] [PubMed] [Google Scholar]
- Fojo A., Akiyama S., Gottesman M. M., Pastan I. Reduced drug accumulation in multiply drug-resistant human KB carcinoma cell lines. Cancer Res. 1985 Jul;45(7):3002–3007. [PubMed] [Google Scholar]
- Ford J. M., Prozialeck W. C., Hait W. N. Structural features determining activity of phenothiazines and related drugs for inhibition of cell growth and reversal of multidrug resistance. Mol Pharmacol. 1989 Jan;35(1):105–115. [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. The multidrug transporter, a double-edged sword. J Biol Chem. 1988 Sep 5;263(25):12163–12166. [PubMed] [Google Scholar]
- Hamada H., Tsuruo T. Purification of the 170- to 180-kilodalton membrane glycoprotein associated with multidrug resistance. 170- to 180-kilodalton membrane glycoprotein is an ATPase. J Biol Chem. 1988 Jan 25;263(3):1454–1458. [PubMed] [Google Scholar]
- Higgins C. F., Gottesman M. M. Is the multidrug transporter a flippase? Trends Biochem Sci. 1992 Jan;17(1):18–21. doi: 10.1016/0968-0004(92)90419-a. [DOI] [PubMed] [Google Scholar]
- Hofsli E., Nissen-Meyer J. Reversal of multidrug resistance by lipophilic drugs. Cancer Res. 1990 Jul 1;50(13):3997–4002. [PubMed] [Google Scholar]
- Horio M., Gottesman M. M., Pastan I. ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proc Natl Acad Sci U S A. 1988 May;85(10):3580–3584. doi: 10.1073/pnas.85.10.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkhandi J., Ferry D. R., Boer R., Gekeler V., Ise W., Kerr D. J. Dexniguldipine-HCl is a potent allosteric inhibitor of [3H]vinblastine binding to P-glycoprotein of CCRF ADR 5000 cells. Eur J Pharmacol. 1994 Dec 15;288(1):105–114. doi: 10.1016/0922-4106(94)90015-9. [DOI] [PubMed] [Google Scholar]
- May G. L., Wright L. C., Dyne M., Mackinnon W. B., Fox R. M., Mountford C. E. Plasma membrane lipid composition of vinblastine sensitive and resistant human leukaemic lymphoblasts. Int J Cancer. 1988 Nov 15;42(5):728–733. doi: 10.1002/ijc.2910420517. [DOI] [PubMed] [Google Scholar]
- McLaughlin S., Eisenberg M. Antibiotics and membrane biology. Annu Rev Biophys Bioeng. 1975;4(00):335–366. doi: 10.1146/annurev.bb.04.060175.002003. [DOI] [PubMed] [Google Scholar]
- Miyamoto K. I., Koga-Takeda K., Koga K., Ohshima T., Nomura M. Saturable function of P-glycoprotein as a drug-efflux pump in multidrug-resistant tumour cells. J Pharm Pharmacol. 1996 May;48(5):522–525. doi: 10.1111/j.2042-7158.1996.tb05966.x. [DOI] [PubMed] [Google Scholar]
- Nooter K., Oostrum R., Jonker R., van Dekken H., Stokdijk W., van den Engh G. Effect of cyclosporin A on daunorubicin accumulation in multidrug-resistant P388 leukemia cells measured by real-time flow cytometry. Cancer Chemother Pharmacol. 1989;23(5):296–300. doi: 10.1007/BF00292407. [DOI] [PubMed] [Google Scholar]
- Pereira E., Borrel M. N., Fiallo M., Garnier-Suillerot A. Non-competitive inhibition of P-glycoprotein-associated efflux of THP-adriamycin by verapamil in living K562 leukemia cells. Biochim Biophys Acta. 1994 Jan 11;1225(2):209–216. doi: 10.1016/0925-4439(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. F., Callaghan R., Ford R. C., Higgins C. F. Structure of the multidrug resistance P-glycoprotein to 2.5 nm resolution determined by electron microscopy and image analysis. J Biol Chem. 1997 Apr 18;272(16):10685–10694. doi: 10.1074/jbc.272.16.10685. [DOI] [PubMed] [Google Scholar]
- Safa A. R., Glover C. J., Meyers M. B., Biedler J. L., Felsted R. L. Vinblastine photoaffinity labeling of a high molecular weight surface membrane glycoprotein specific for multidrug-resistant cells. J Biol Chem. 1986 May 15;261(14):6137–6140. [PubMed] [Google Scholar]
- Saitoh H., Kawai S., Iseki K., Miyazaki K., Arita T. Transport characteristics of [3H]-chlorpromazine across rat small intestinal brush border membrane. J Pharm Pharmacol. 1989 Mar;41(3):200–202. doi: 10.1111/j.2042-7158.1989.tb06431.x. [DOI] [PubMed] [Google Scholar]
- Sehested M., Skovsgaard T., Jensen P. B., Demant E. J., Friche E., Bindslev N. Transport of the multidrug resistance modulators verapamil and azidopine in wild type and daunorubicin resistant Ehrlich ascites tumour cells. Br J Cancer. 1990 Jul;62(1):37–41. doi: 10.1038/bjc.1990.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H., Ghishan F. K. Intestinal calcium transport in spontaneously hypertensive rats (SHR) and their genetically matched WKY rats. Proc Soc Exp Biol Med. 1990 May;194(1):26–31. doi: 10.3181/00379727-194-43049. [DOI] [PubMed] [Google Scholar]
- Spoelstra E. C., Westerhoff H. V., Dekker H., Lankelma J. Kinetics of daunorubicin transport by P-glycoprotein of intact cancer cells. Eur J Biochem. 1992 Jul 15;207(2):567–579. doi: 10.1111/j.1432-1033.1992.tb17083.x. [DOI] [PubMed] [Google Scholar]
- Stein W. D. Kinetics of the multidrug transporter (P-glycoprotein) and its reversal. Physiol Rev. 1997 Apr;77(2):545–590. doi: 10.1152/physrev.1997.77.2.545. [DOI] [PubMed] [Google Scholar]
- Syed S. K., Christopherson R. I., Roufogalis B. D. Chlorpromazine transport in membrane vesicles from multidrug resistant CCRF-CEM cells. Biochem Mol Biol Int. 1996 Jul;39(4):687–696. doi: 10.1080/15216549600201761. [DOI] [PubMed] [Google Scholar]
- Syed S. K., Christopherson R. I., Roufogalis B. D. Vinblastine transport by membrane vesicles from human multidrug-resistant CCRF-CEM leukaemia cells: inhibition by taxol and membrane permeabilising agents. Biochem Mol Biol Int. 1993 Jul;30(4):743–753. [PubMed] [Google Scholar]
- Tamai I., Safa A. R. Azidopine noncompetitively interacts with vinblastine and cyclosporin A binding to P-glycoprotein in multidrug resistant cells. J Biol Chem. 1991 Sep 5;266(25):16796–16800. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982 Nov;42(11):4730–4733. [PubMed] [Google Scholar]


