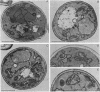
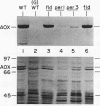
Abstract
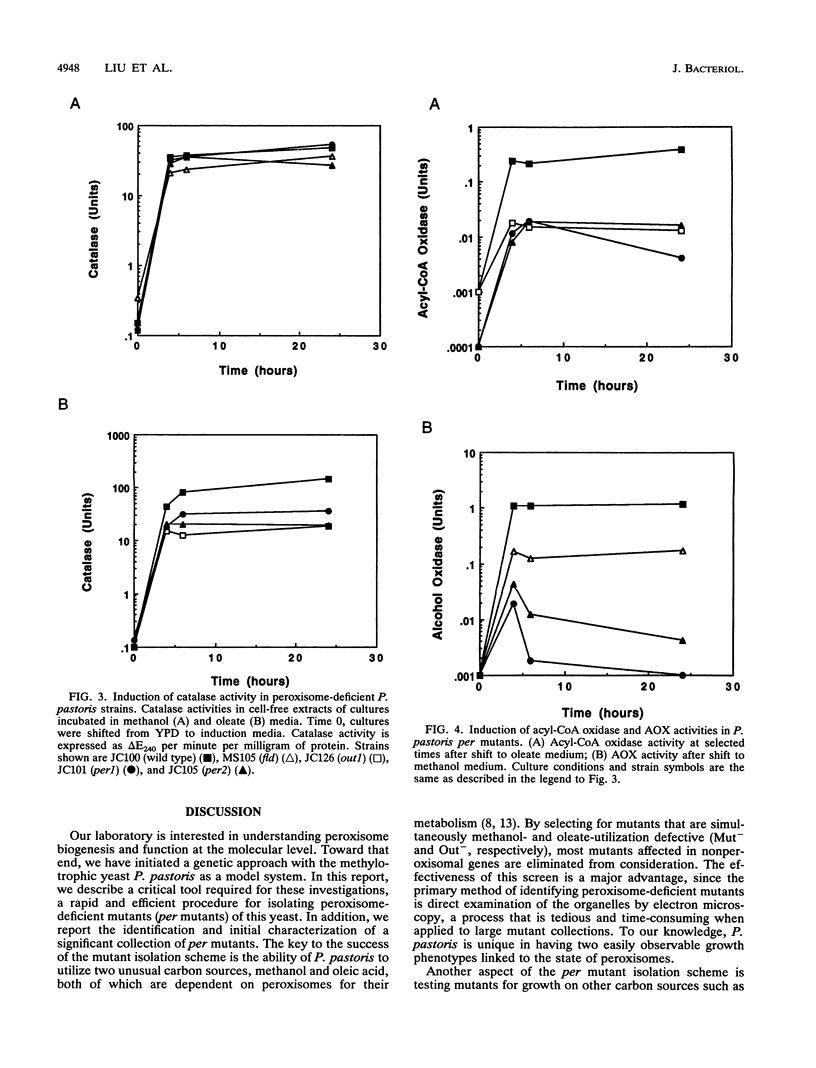
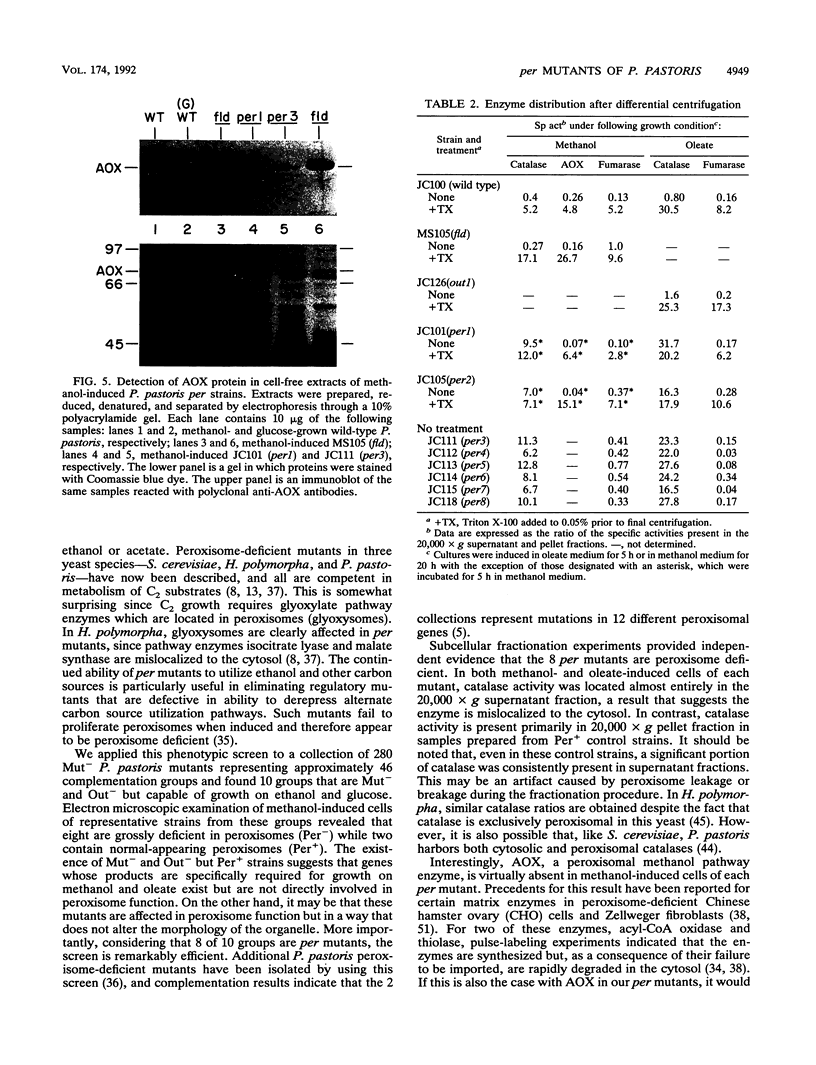
We describe a rapid and efficient screen for peroxisome-deficient (per) mutants in the yeast Pichia pastoris. The screen relies on the unusual ability of P. pastoris to grow on two carbon sources, methanol and oleic acid, both of which absolutely require peroxisomes to be metabolized. A collection of 280 methanol utilization-defective (Mut-) P. pastoris mutants was isolated, organized into 46 complementation groups, and tested for those that were also oleate-utilization defective (Out-) but still capable of growth on ethanol and glucose. Mutants in 10 groups met this phenotypic description, and 8 of these were observed by electron microscopy to be peroxisome deficient (Per-). In each per mutant, Mut-, Out-, and Per- phenotypes were tightly linked and therefore were most likely due to a mutation at a single locus. Subcellular fractionation experiments indicated that the peroxisomal marker enzyme catalase was mislocalized to the cytosol in both methanol- and oleate-induced cultures of the mutants. In contrast, alcohol oxidase, a peroxisomal methanol utilization pathway enzyme, was virtually absent from per mutant cells. The relative ease of per mutant isolation in P. pastoris, in conjunction with well-developed procedures for its molecular and genetic manipulation, makes this organism an attractive system for studies on peroxisome biogenesis.
Full text
PDF


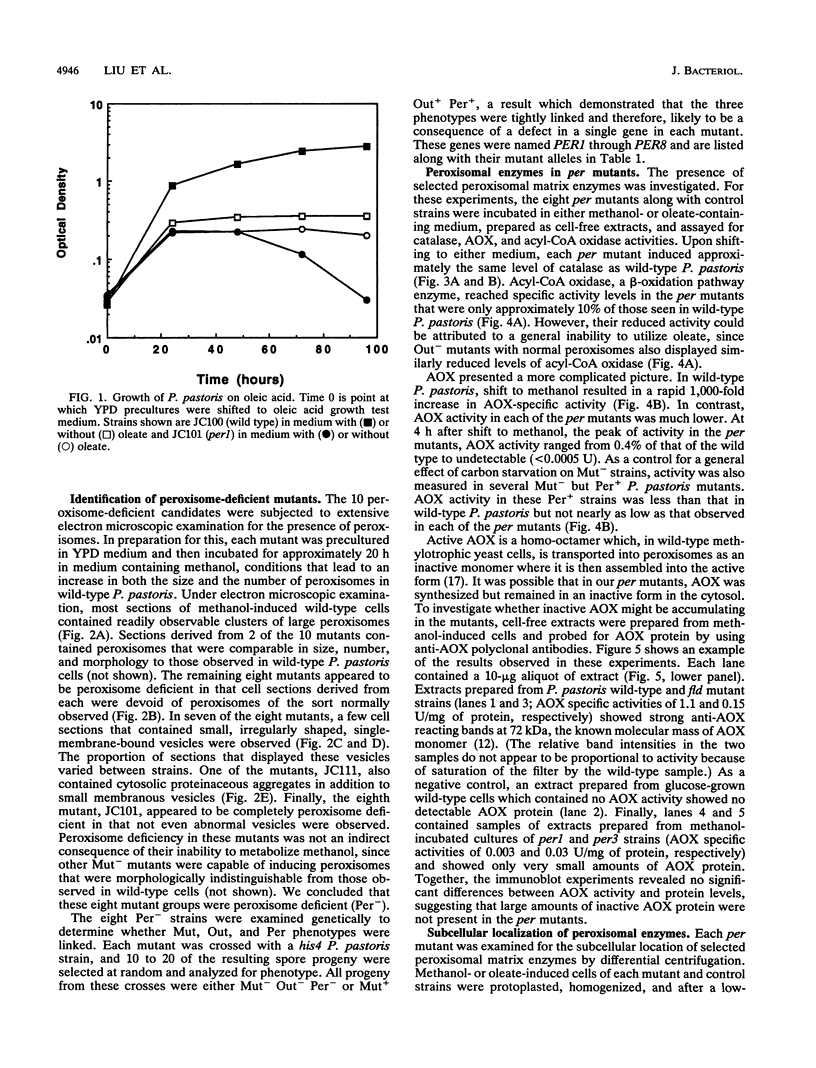
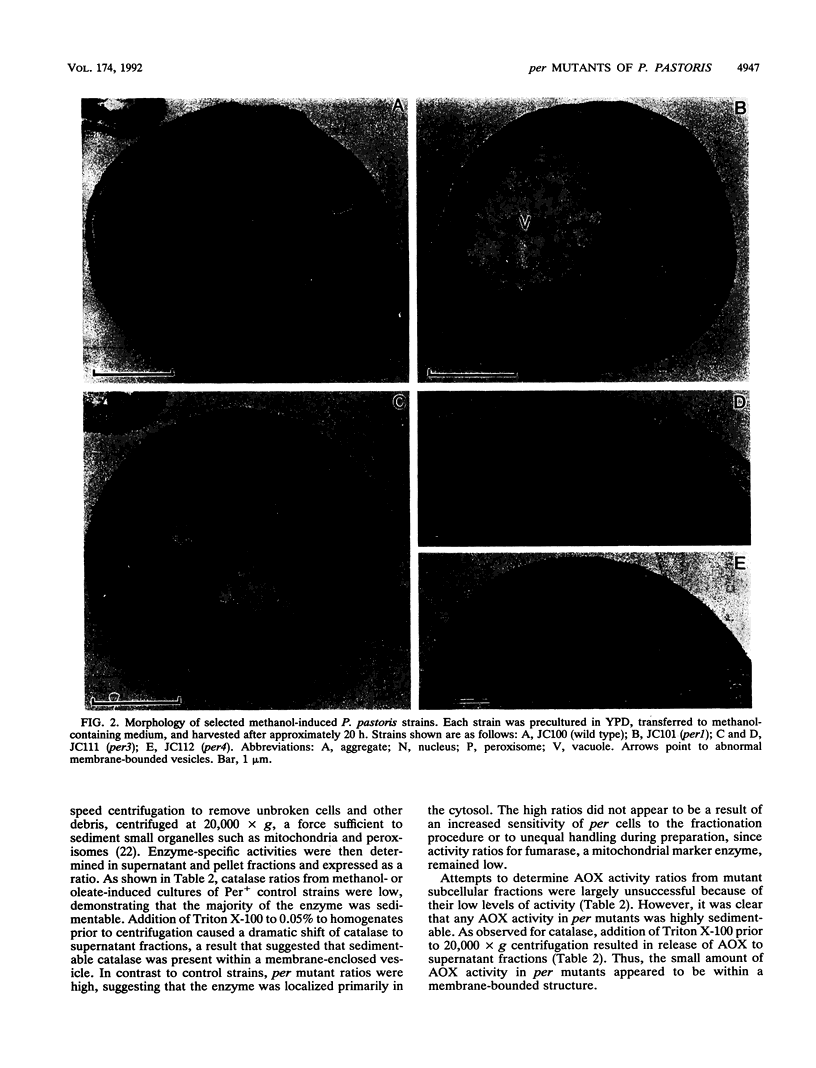


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borst P. Peroxisome biogenesis revisited. Biochim Biophys Acta. 1989 Jun 1;1008(1):1–13. doi: 10.1016/0167-4781(89)90163-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brul S., Westerveld A., Strijland A., Wanders R. J., Schram A. W., Heymans H. S., Schutgens R. B., van den Bosch H., Tager J. M. Genetic heterogeneity in the cerebrohepatorenal (Zellweger) syndrome and other inherited disorders with a generalized impairment of peroxisomal functions. A study using complementation analysis. J Clin Invest. 1988 Jun;81(6):1710–1715. doi: 10.1172/JCI113510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg J. M., Barringer K. J., Hessler A. Y., Madden K. R. Pichia pastoris as a host system for transformations. Mol Cell Biol. 1985 Dec;5(12):3376–3385. doi: 10.1128/mcb.5.12.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg J. M., Madden K. R., Barringer K. J., Thill G. P., Stillman C. A. Functional characterization of the two alcohol oxidase genes from the yeast Pichia pastoris. Mol Cell Biol. 1989 Mar;9(3):1316–1323. doi: 10.1128/mcb.9.3.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommes V., Baumgart C., Kunau W. H. Degradation of unsaturated fatty acids in peroxisomes. Existence of a 2,4-dienoyl-CoA reductase pathway. J Biol Chem. 1981 Aug 25;256(16):8259–8262. [PubMed] [Google Scholar]
- Ellis S. B., Brust P. F., Koutz P. J., Waters A. F., Harpold M. M., Gingeras T. R. Isolation of alcohol oxidase and two other methanol regulatable genes from the yeast Pichia pastoris. Mol Cell Biol. 1985 May;5(5):1111–1121. doi: 10.1128/mcb.5.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Rachubinski R. A., Lazarow P. B. Synthesis of a major integral membrane polypeptide of rat liver peroxisomes on free polysomes. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7127–7131. doi: 10.1073/pnas.81.22.7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfischer S., Moore C. L., Johnson A. B., Spiro A. J., Valsamis M. P., Wisniewski H. K., Ritch R. H., Norton W. T., Rapin I., Gartner L. M. Peroxisomal and mitochondrial defects in the cerebro-hepato-renal syndrome. Science. 1973 Oct 5;182(4107):62–64. doi: 10.1126/science.182.4107.62. [DOI] [PubMed] [Google Scholar]
- Goodman J. M., Scott C. W., Donahue P. N., Atherton J. P. Alcohol oxidase assembles post-translationally into the peroxisome of Candida boidinii. J Biol Chem. 1984 Jul 10;259(13):8485–8493. [PubMed] [Google Scholar]
- Gould S. G., Keller G. A., Subramani S. Identification of a peroxisomal targeting signal at the carboxy terminus of firefly luciferase. J Cell Biol. 1987 Dec;105(6 Pt 2):2923–2931. doi: 10.1083/jcb.105.6.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Krisans S., Keller G. A., Subramani S. Antibodies directed against the peroxisomal targeting signal of firefly luciferase recognize multiple mammalian peroxisomal proteins. J Cell Biol. 1990 Jan;110(1):27–34. doi: 10.1083/jcb.110.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaka T., Small G. M., Lazarow P. B. Translocation of acyl-CoA oxidase into peroxisomes requires ATP hydrolysis but not a membrane potential. J Cell Biol. 1987 Dec;105(6 Pt 2):2915–2922. doi: 10.1083/jcb.105.6.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiryo T., Abe M., Okazaki K., Kato S., Shimamoto N. Absence of DNA in peroxisomes of Candida tropicalis. J Bacteriol. 1982 Oct;152(1):269–274. doi: 10.1128/jb.152.1.269-274.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G. A., Krisans S., Gould S. J., Sommer J. M., Wang C. C., Schliebs W., Kunau W., Brody S., Subramani S. Evolutionary conservation of a microbody targeting signal that targets proteins to peroxisomes, glyoxysomes, and glycosomes. J Cell Biol. 1991 Sep;114(5):893–904. doi: 10.1083/jcb.114.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y., Small G. M., Watkins P., Moser H. Presence of the peroxisomal 22-kDa integral membrane protein in the liver of a person lacking recognizable peroxisomes (Zellweger syndrome). Proc Natl Acad Sci U S A. 1986 Dec;83(23):9193–9196. doi: 10.1073/pnas.83.23.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell S. J., Stewart L. C., Talin A., Yaffe M. P. Temperature-sensitive yeast mutants defective in mitochondrial inheritance. J Cell Biol. 1990 Sep;111(3):967–976. doi: 10.1083/jcb.111.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolay K., Veenhuis M., Douma A. C., Harder W. A 31P NMR study of the internal pH of yeast peroxisomes. Arch Microbiol. 1987 Feb;147(1):37–41. doi: 10.1007/BF00492902. [DOI] [PubMed] [Google Scholar]
- Osumi T., Tsukamoto T., Hata S., Yokota S., Miura S., Fujiki Y., Hijikata M., Miyazawa S., Hashimoto T. Amino-terminal presequence of the precursor of peroxisomal 3-ketoacyl-CoA thiolase is a cleavable signal peptide for peroxisomal targeting. Biochem Biophys Res Commun. 1991 Dec 31;181(3):947–954. doi: 10.1016/0006-291x(91)92028-i. [DOI] [PubMed] [Google Scholar]
- Roscher A. A., Hoefler S., Hoefler G., Paschke E., Paltauf F., Moser A., Moser H. Genetic and phenotypic heterogeneity in disorders of peroxisome biogenesis--a complementation study involving cell lines from 19 patients. Pediatr Res. 1989 Jul;26(1):67–72. doi: 10.1203/00006450-198907000-00019. [DOI] [PubMed] [Google Scholar]
- Santos M. J., Imanaka T., Shio H., Lazarow P. B. Peroxisomal integral membrane proteins in control and Zellweger fibroblasts. J Biol Chem. 1988 Jul 25;263(21):10502–10509. [PubMed] [Google Scholar]
- Santos M. J., Imanaka T., Shio H., Small G. M., Lazarow P. B. Peroxisomal membrane ghosts in Zellweger syndrome--aberrant organelle assembly. Science. 1988 Mar 25;239(4847):1536–1538. doi: 10.1126/science.3281254. [DOI] [PubMed] [Google Scholar]
- Schram A. W., Strijland A., Hashimoto T., Wanders R. J., Schutgens R. B., van den Bosch H., Tager J. M. Biosynthesis and maturation of peroxisomal beta-oxidation enzymes in fibroblasts in relation to the Zellweger syndrome and infantile Refsum disease. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6156–6158. doi: 10.1073/pnas.83.16.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M., Binder M., Adam G., Hartig A., Ruis H. Control of peroxisome proliferation in Saccharomyces cerevisiae by ADR1, SNF1 (CAT1, CCR1) and SNF4 (CAT3). Yeast. 1992 Apr;8(4):303–309. doi: 10.1002/yea.320080407. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Orii T., Mori M., Tatibana M., Hashimoto T. Deficient activities and proteins of peroxisomal beta-oxidation enzymes in infants with Zellweger syndrome. Clin Chim Acta. 1986 Apr 30;156(2):191–196. doi: 10.1016/0009-8981(86)90152-x. [DOI] [PubMed] [Google Scholar]
- Swinkels B. W., Gould S. J., Bodnar A. G., Rachubinski R. A., Subramani S. A novel, cleavable peroxisomal targeting signal at the amino-terminus of the rat 3-ketoacyl-CoA thiolase. EMBO J. 1991 Nov;10(11):3255–3262. doi: 10.1002/j.1460-2075.1991.tb04889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E. Isolation of subcellular organelles of metabolism on isopycnic sucrose gradients. Methods Enzymol. 1974;31:734–746. doi: 10.1016/0076-6879(74)31077-4. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T., Yokota S., Fujiki Y. Isolation and characterization of Chinese hamster ovary cell mutants defective in assembly of peroxisomes. J Cell Biol. 1990 Mar;110(3):651–660. doi: 10.1083/jcb.110.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Mozaffar S., Tanaka A. Catalase from Candida boidinii 2201. Methods Enzymol. 1990;188:463–467. doi: 10.1016/0076-6879(90)88074-k. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., Mateblowski M., Kunau W. H., Harder W. Proliferation of microbodies in Saccharomyces cerevisiae. Yeast. 1987 Jun;3(2):77–84. doi: 10.1002/yea.320030204. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., van Dijken J. P., Pilon S. A., Harder W. Development of crystalline peroxisomes in methanol-grown cells of the yeast Hansenula polymorpha and its relation to environmental conditions. Arch Microbiol. 1978 May 30;117(2):153–163. doi: 10.1007/BF00402303. [DOI] [PubMed] [Google Scholar]
- Walton P. A., Gould S. J., Feramisco J. R., Subramani S. Transport of microinjected proteins into peroxisomes of mammalian cells: inability of Zellweger cell lines to import proteins with the SKL tripeptide peroxisomal targeting signal. Mol Cell Biol. 1992 Feb;12(2):531–541. doi: 10.1128/mcb.12.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. N. Structure-function relationships in the peroxisome: implications for human disease. Biochem Med Metab Biol. 1991 Dec;46(3):288–298. doi: 10.1016/0885-4505(91)90079-z. [DOI] [PubMed] [Google Scholar]
- Zoeller R. A., Allen L. A., Santos M. J., Lazarow P. B., Hashimoto T., Tartakoff A. M., Raetz C. R. Chinese hamster ovary cell mutants defective in peroxisome biogenesis. Comparison to Zellweger syndrome. J Biol Chem. 1989 Dec 25;264(36):21872–21878. [PubMed] [Google Scholar]
- Zoeller R. A., Raetz C. R. Isolation of animal cell mutants deficient in plasmalogen biosynthesis and peroxisome assembly. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5170–5174. doi: 10.1073/pnas.83.14.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijken J. P., Veenhuis M., Vermeulen C. A., Harder W. Cytochemical localization of catalase activity in methanol-grown Hansenula polymorpha. Arch Microbiol. 1975 Nov 7;105(3):261–267. doi: 10.1007/BF00447145. [DOI] [PubMed] [Google Scholar]
- van der Klei I. J., Bystrykh L. V., Harder W. Alcohol oxidase from Hansenula polymorpha CBS 4732. Methods Enzymol. 1990;188:420–427. doi: 10.1016/0076-6879(90)88067-k. [DOI] [PubMed] [Google Scholar]