Abstract
Midkine (MK) is a growth factor identified as a product of a retinoic acid-responsive gene. A truncated form of MK mRNA, which lacks a sequence encoding the N-terminally located domain, was recently found in cancer cells. We investigated the expression of the truncated MK mRNA in specimens of 47 surgically removed human gastrointestinal organs using polymerase chain reaction. Truncated MK was not detected in all of the 46 corresponding non-cancerous regions. On the other hand, this short MK mRNA was expressed in the primary tumours in 12 of 16 gastric cancers, 8 of 13 colorectal carcinomas, five of nine hepatocellular carcinomas, two of two oesophageal carcinomas and one ampullary duodenal cancer. In addition, truncated MK was detectable in all of the 14 lymph node metastases but in none of three metastatic sites in the liver, suggesting that truncated MK mRNA could become a good marker of nodal metastases in gastrointestinal tract.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi Y., Matsubara S., Pedraza C., Ozawa M., Tsutsui J., Takamatsu H., Noguchi H., Akiyama T., Muramatsu T. Midkine as a novel target gene for the Wilms' tumor suppressor gene (WT1). Oncogene. 1996 Nov 21;13(10):2197–2203. [PubMed] [Google Scholar]
- Arcari P., Martinelli R., Salvatore F. The complete sequence of a full length cDNA for human liver glyceraldehyde-3-phosphate dehydrogenase: evidence for multiple mRNA species. Nucleic Acids Res. 1984 Dec 11;12(23):9179–9189. doi: 10.1093/nar/12.23.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
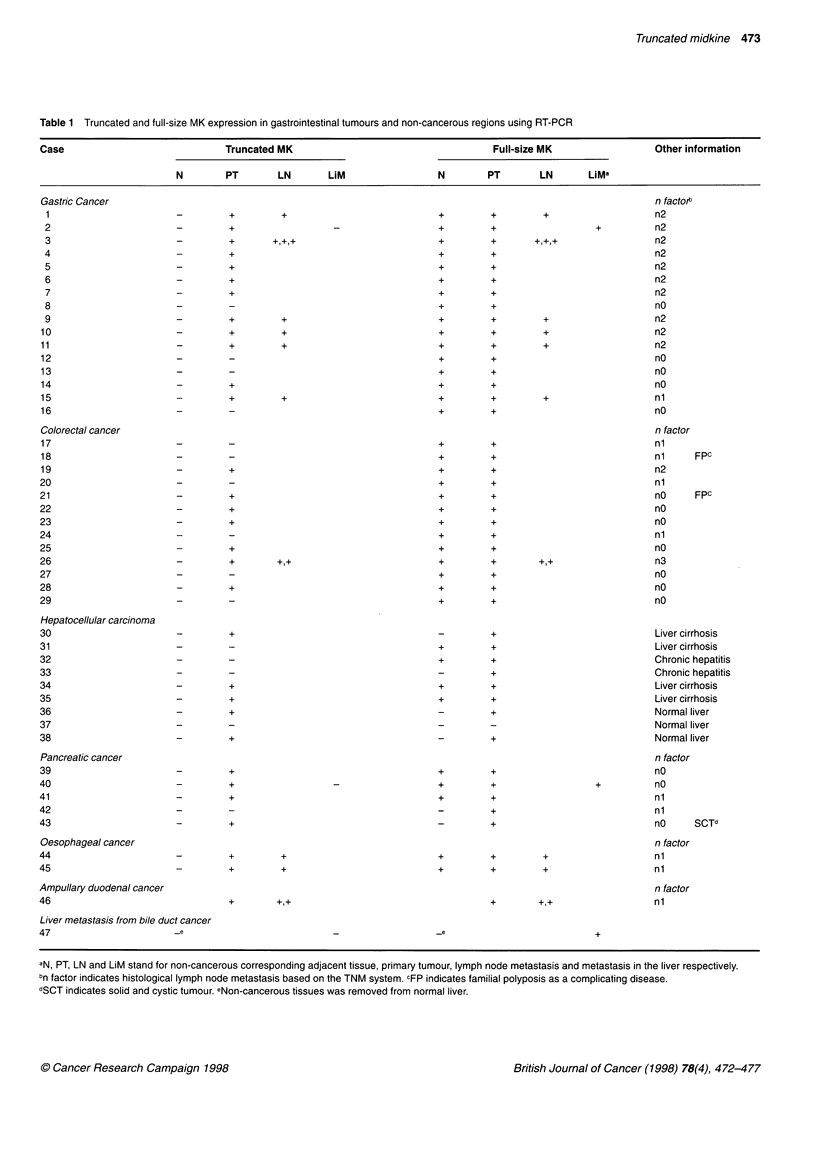
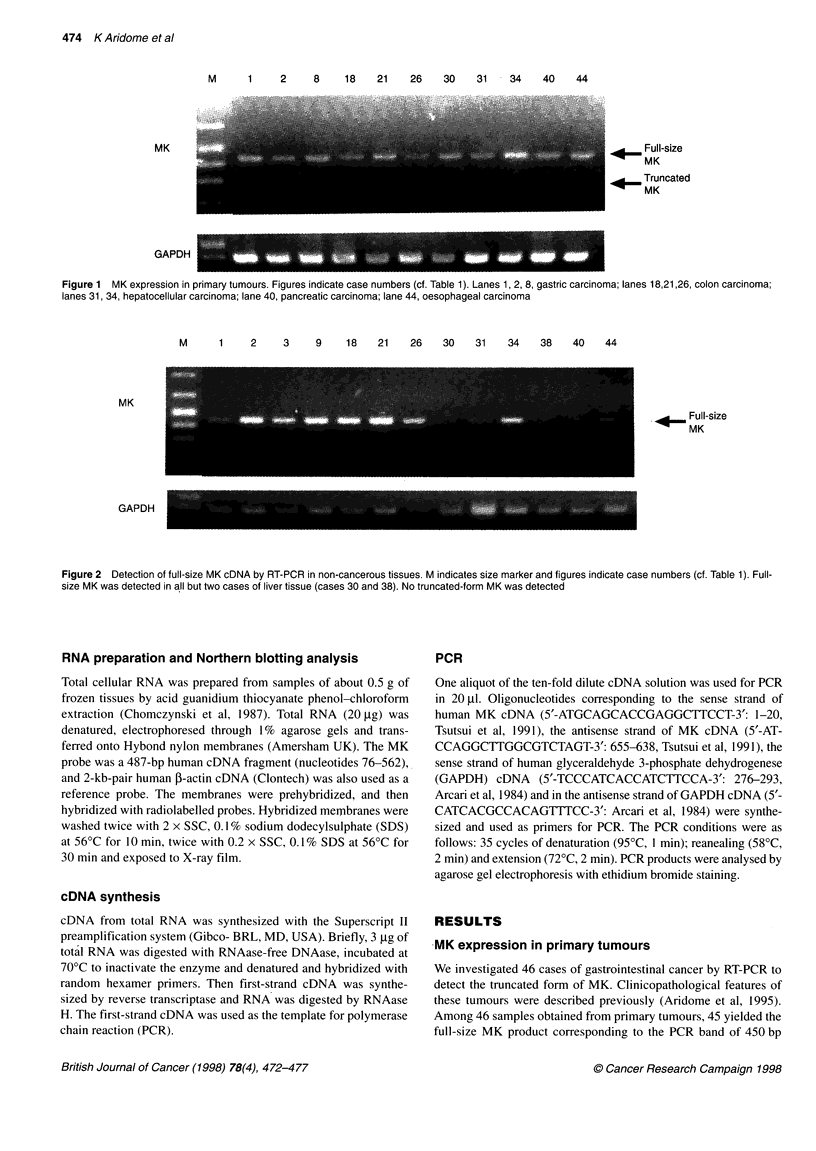
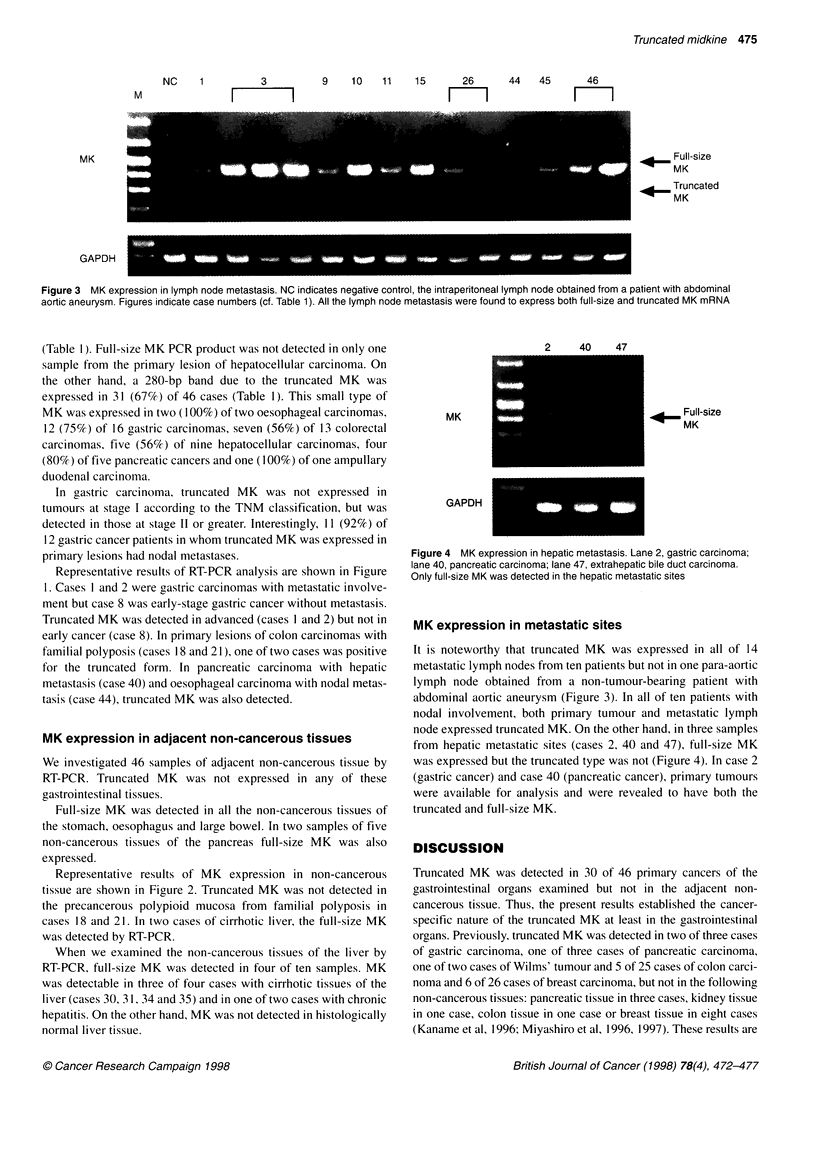
- Aridome K., Tsutsui J., Takao S., Kadomatsu K., Ozawa M., Aikou T., Muramatsu T. Increased midkine gene expression in human gastrointestinal cancers. Jpn J Cancer Res. 1995 Jul;86(7):655–661. doi: 10.1111/j.1349-7006.1995.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A. K., Li Y. S., Deuel T. F. Pleiotrophin transforms NIH 3T3 cells and induces tumors in nude mice. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):679–682. doi: 10.1073/pnas.90.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Choudhuri R., Zhang H. T., Donnini S., Ziche M., Bicknell R. An angiogenic role for the neurokines midkine and pleiotrophin in tumorigenesis. Cancer Res. 1997 May 1;57(9):1814–1819. [PubMed] [Google Scholar]
- Czubayko F., Schulte A. M., Berchem G. J., Wellstein A. Melanoma angiogenesis and metastasis modulated by ribozyme targeting of the secreted growth factor pleiotrophin. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14753–14758. doi: 10.1073/pnas.93.25.14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver R. I., Jr, Chan C. S., Milner P. G. Reciprocal expression of pleiotrophin and midkine in normal versus malignant lung tissues. Am J Respir Cell Mol Biol. 1993 Nov;9(5):463–466. doi: 10.1165/ajrcmb/9.5.463. [DOI] [PubMed] [Google Scholar]
- Garver R. I., Jr, Radford D. M., Donis-Keller H., Wick M. R., Milner P. G. Midkine and pleiotrophin expression in normal and malignant breast tissue. Cancer. 1994 Sep 1;74(5):1584–1590. doi: 10.1002/1097-0142(19940901)74:5<1584::aid-cncr2820740514>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Hayashi N., Arakawa H., Nagase H., Yanagisawa A., Kato Y., Ohta H., Takano S., Ogawa M., Nakamura Y. Genetic diagnosis identifies occult lymph node metastases undetectable by the histopathological method. Cancer Res. 1994 Jul 15;54(14):3853–3856. [PubMed] [Google Scholar]
- Hayashi N., Ito I., Yanagisawa A., Kato Y., Nakamori S., Imaoka S., Watanabe H., Ogawa M., Nakamura Y. Genetic diagnosis of lymph-node metastasis in colorectal cancer. Lancet. 1995 May 20;345(8960):1257–1259. doi: 10.1016/s0140-6736(95)90922-2. [DOI] [PubMed] [Google Scholar]
- Inoue S., Nakao A., Kasai Y., Harada A., Nonami T., Takagi H. Detection of hepatic micrometastasis in pancreatic adenocarcinoma patients by two-stage polymerase chain reaction/restriction fragment length polymorphism analysis. Jpn J Cancer Res. 1995 Jul;86(7):626–630. doi: 10.1111/j.1349-7006.1995.tb02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadomatsu K., Tomomura M., Muramatsu T. cDNA cloning and sequencing of a new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid-gestation period of mouse embryogenesis. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1312–1318. doi: 10.1016/s0006-291x(88)80505-9. [DOI] [PubMed] [Google Scholar]
- Kaname T., Kadomatsu K., Aridome K., Yamashita S., Sakamoto K., Ogawa M., Muramatsu T., Yamamura K. The expression of truncated MK in human tumors. Biochem Biophys Res Commun. 1996 Feb 6;219(1):256–260. doi: 10.1006/bbrc.1996.0214. [DOI] [PubMed] [Google Scholar]
- Kojima S., Inui T., Kimura T., Sakakibara S., Muramatsu H., Amanuma H., Maruta H., Muramatsu T. Synthetic peptides derived from midkine enhance plasminogen activator activity in bovine aortic endothelial cells. Biochem Biophys Res Commun. 1995 Jan 17;206(2):468–473. doi: 10.1006/bbrc.1995.1066. [DOI] [PubMed] [Google Scholar]
- Kojima S., Muramatsu H., Amanuma H., Muramatsu T. Midkine enhances fibrinolytic activity of bovine endothelial cells. J Biol Chem. 1995 Apr 21;270(16):9590–9596. doi: 10.1074/jbc.270.16.9590. [DOI] [PubMed] [Google Scholar]
- Miyashiro I., Kaname T., Nakayama T., Nakamori S., Yagyu T., Monden T., Kikkawa N., Nishisho I., Muramatsu T., Monden M. Expression of truncated midkine in human colorectal cancers. Cancer Lett. 1996 Sep 10;106(2):287–291. doi: 10.1016/0304-3835(96)04333-9. [DOI] [PubMed] [Google Scholar]
- Miyashiro I., Kaname T., Shin E., Wakasugi E., Monden T., Takatsuka Y., Kikkawa N., Muramatsu T., Monden M., Akiyama T. Midkine expression in human breast cancers: expression of truncated form. Breast Cancer Res Treat. 1997 Mar;43(1):1–6. doi: 10.1023/a:1005748728351. [DOI] [PubMed] [Google Scholar]
- Mori M., Mimori K., Inoue H., Barnard G. F., Tsuji K., Nanbara S., Ueo H., Akiyoshi T. Detection of cancer micrometastases in lymph nodes by reverse transcriptase-polymerase chain reaction. Cancer Res. 1995 Aug 1;55(15):3417–3420. [PubMed] [Google Scholar]
- Muramatsu H., Inui T., Kimura T., Sakakibara S., Song X. J., Maruta H., Muramatsu T. Localization of heparin-binding, neurite outgrowth and antigenic regions in midkine molecule. Biochem Biophys Res Commun. 1994 Sep 15;203(2):1131–1139. doi: 10.1006/bbrc.1994.2300. [DOI] [PubMed] [Google Scholar]
- Muramatsu H., Muramatsu T. Purification of recombinant midkine and examination of its biological activities: functional comparison of new heparin binding factors. Biochem Biophys Res Commun. 1991 Jun 14;177(2):652–658. doi: 10.1016/0006-291x(91)91838-4. [DOI] [PubMed] [Google Scholar]
- Muramatsu T. Midkine (MK), the product of a retinoic acid responsive gene, and pleiotrophin constitute a new protein family regulating growth and differentiation. Int J Dev Biol. 1993 Mar;37(1):183–188. [PubMed] [Google Scholar]
- Nakagawara A., Milbrandt J., Muramatsu T., Deuel T. F., Zhao H., Cnaan A., Brodeur G. M. Differential expression of pleiotrophin and midkine in advanced neuroblastomas. Cancer Res. 1995 Apr 15;55(8):1792–1797. [PubMed] [Google Scholar]
- O'Brien T., Cranston D., Fuggle S., Bicknell R., Harris A. L. The angiogenic factor midkine is expressed in bladder cancer, and overexpression correlates with a poor outcome in patients with invasive cancers. Cancer Res. 1996 Jun 1;56(11):2515–2518. [PubMed] [Google Scholar]
- Schulte A. M., Lai S., Kurtz A., Czubayko F., Riegel A. T., Wellstein A. Human trophoblast and choriocarcinoma expression of the growth factor pleiotrophin attributable to germ-line insertion of an endogenous retrovirus. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14759–14764. doi: 10.1073/pnas.93.25.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomomura M., Kadomatsu K., Matsubara S., Muramatsu T. A retinoic acid-responsive gene, MK, found in the teratocarcinoma system. Heterogeneity of the transcript and the nature of the translation product. J Biol Chem. 1990 Jun 25;265(18):10765–10770. [PubMed] [Google Scholar]
- Tsutsui J., Kadomatsu K., Matsubara S., Nakagawara A., Hamanoue M., Takao S., Shimazu H., Ohi Y., Muramatsu T. A new family of heparin-binding growth/differentiation factors: increased midkine expression in Wilms' tumor and other human carcinomas. Cancer Res. 1993 Mar 15;53(6):1281–1285. [PubMed] [Google Scholar]
- Tsutsui J., Uehara K., Kadomatsu K., Matsubara S., Muramatsu T. A new family of heparin-binding factors: strong conservation of midkine (MK) sequences between the human and the mouse. Biochem Biophys Res Commun. 1991 Apr 30;176(2):792–797. doi: 10.1016/s0006-291x(05)80255-4. [DOI] [PubMed] [Google Scholar]