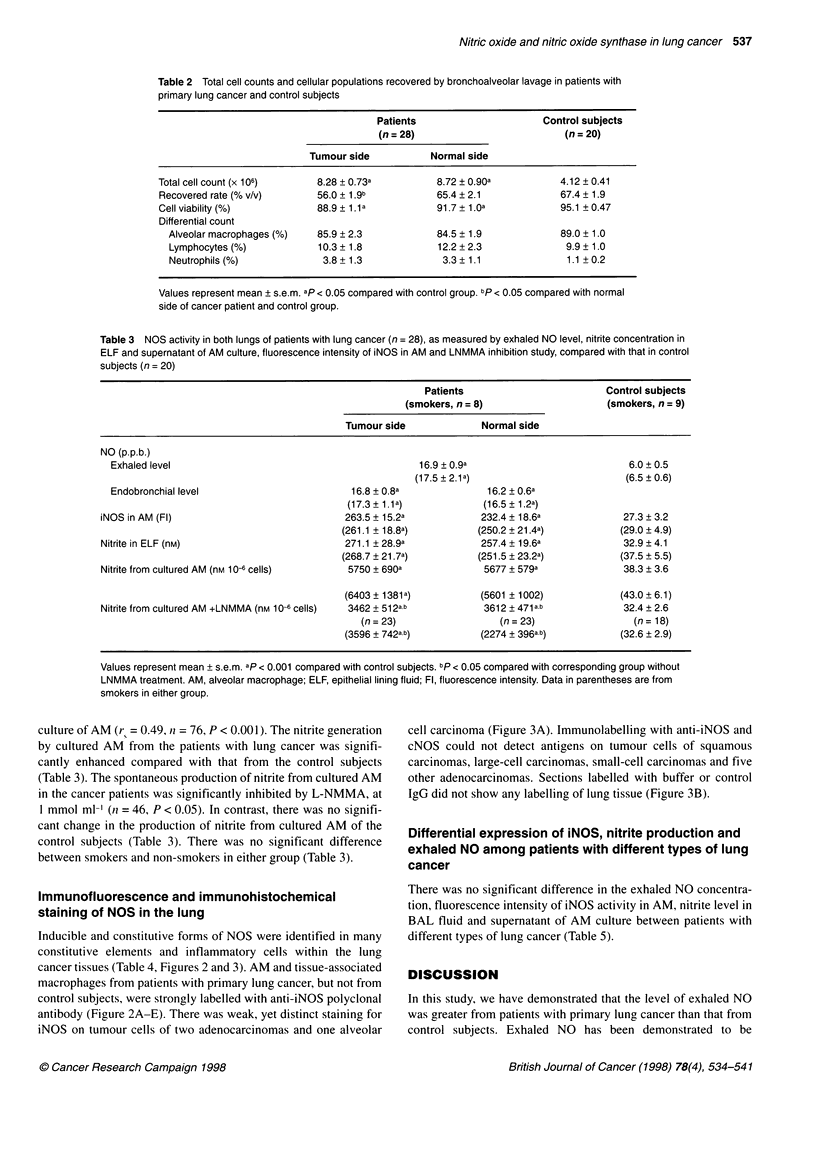
Abstract
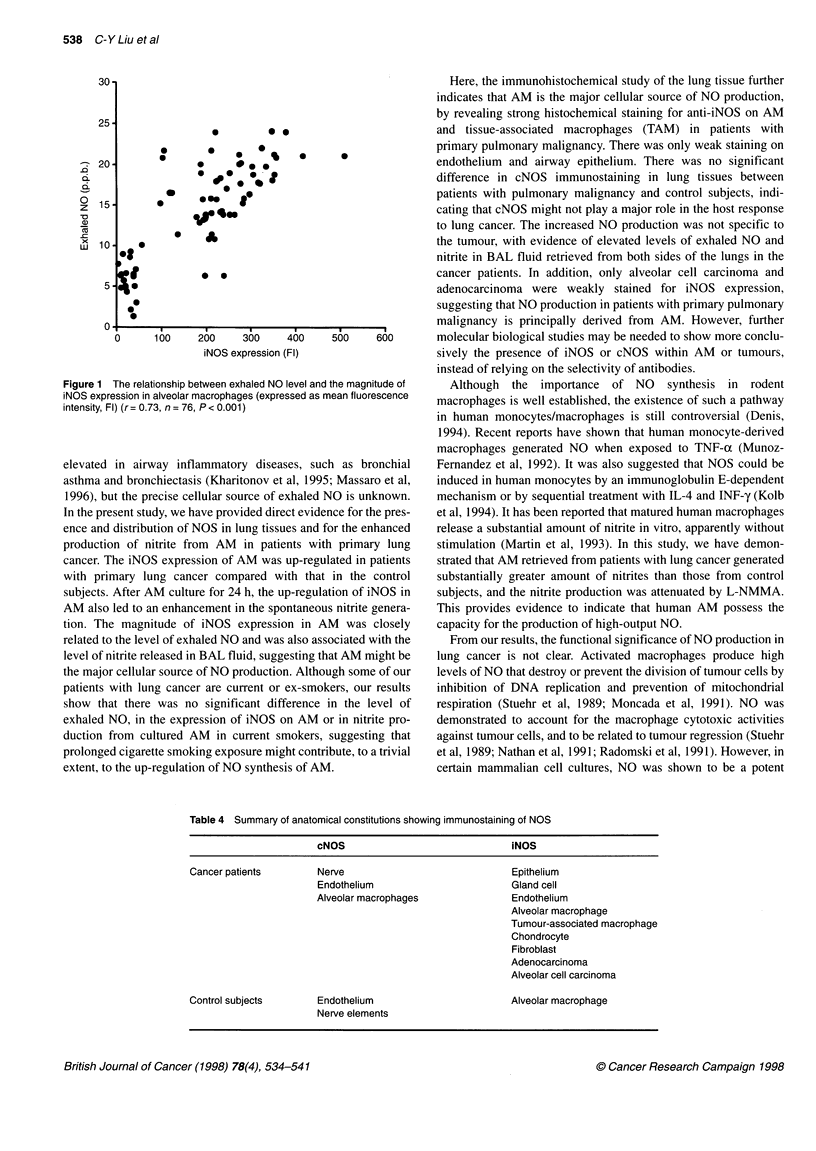
Monocyte-macrophage series have an important role in host surveillance against cancer. The cytotoxic/cytostatic activity of macrophages is, to a great extent, attributed to the up-regulation of inducible nitric oxide synthase (iNOS) and production of nitric oxide (NO). Here, in 28 patients with primary lung cancer and 20 control subjects, we measured the concentration of exhaled NO and nitrite in epithelial lining fluid (ELF) using a chemiluminescence NO analyser, and studied NOS expression in alveolar macrophages (AM) and lung tissues by flow cytometry; immunohistochemical analysis was also undertaken. The mean fluorescence intensity (FI) of iNOS expression in AM was significantly increased in patients with lung cancer (tumour side 263.5 +/- 15.2 FI, normal side 232.4 +/- 18.6 FI; n = 28) compared with that in control subjects (27.3 +/- 3.2 FI; n = 20, P< 0.001). The level of exhaled NO from cancer patients (16.9 +/- 0.9 p.p.b.; n = 28) was significantly higher than that in the control group (6.0 +/- 0.5 p.p.b.; n = 20, P < 0.001). The level of nitrite was also significantly higher in ELF from cancer patients (tumour side 271.1 +/- 28.9 nM and normal side 257.4 +/- 19.6 nM vs control subjects 32.9 +/- 4.1 nM; P< 0.001). The intensity of iNOS expression in AM was correlated with the level of exhaled NO (rs = 0.73, n = 76, P< 0.001) and the nitrite released in ELF (rs = 0.56, n = 76, P< 0.001). The nitrite generation of cultured AM from patients with lung cancer was significantly enhanced compared with that of control subjects after culture for 24 h (tumour side 5.75 +/- 0.69 and normal side 5.68 +/- 0.58 microM per 106 cells vs control group 38.3 +/- 3.6 nM per 106 cells; P< 0.001). The distribution of iNOS was identified in AM, tumour-associated macrophages, endothelium, chondrocytes, airway epithelium of both lungs and malignant cells (adenocarcinoma and alveolar cell carcinoma) of cancer patients. cNOS was labelled in alveolar macrophages, endothelial cells and nerve elements from lung tissue. Our results indicate that, in patients with primary lung cancer, the production of NO from alveolar macrophages was increased as a result of the up-regulation of iNOS activity. The increased NO production was not specific to the tumour side and might be attributed to the tumour-associated non-specific immunological and inflammatory processes of the host.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams J., Doyle L. A., Aisner J. Staging, prognostic factors, and special considerations in small cell lung cancer. Semin Oncol. 1988 Jun;15(3):261–277. [PubMed] [Google Scholar]
- Adams D. O., Kao K. J., Farb R., Pizzo S. V. Effector mechanisms of cytolytically activated macrophages. II. Secretion of a cytolytic factor by activated macrophages and its relationship to secreted neutral proteases. J Immunol. 1980 Jan;124(1):293–300. [PubMed] [Google Scholar]
- Alleva D. G., Burger C. J., Elgert K. D. Tumor-induced regulation of suppressor macrophage nitric oxide and TNF-alpha production. Role of tumor-derived IL-10, TGF-beta, and prostaglandin E2. J Immunol. 1994 Aug 15;153(4):1674–1686. [PubMed] [Google Scholar]
- Arias-Díaz J., Vara E., Torres-Melero J., García C., Baki W., Ramírez-Armengol J. A., Balibrea J. L. Nitrite/nitrate and cytokine levels in bronchoalveolar lavage fluid of lung cancer patients. Cancer. 1994 Sep 1;74(5):1546–1551. doi: 10.1002/1097-0142(19940901)74:5<1546::aid-cncr2820740509>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Bucana C. D., Hoyer L. C., Schroit A. J., Kleinerman E., Fidler I. J. Ultrastructural studies of the interaction between liposome-activated human blood monocytes and allogeneic tumor cells in vitro. Am J Pathol. 1983 Jul;112(1):101–111. [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Denis M. Human monocytes/macrophages: NO or no NO? J Leukoc Biol. 1994 May;55(5):682–684. doi: 10.1002/jlb.55.5.682. [DOI] [PubMed] [Google Scholar]
- Dong Z., Staroselsky A. H., Qi X., Xie K., Fidler I. J. Inverse correlation between expression of inducible nitric oxide synthase activity and production of metastasis in K-1735 murine melanoma cells. Cancer Res. 1994 Feb 1;54(3):789–793. [PubMed] [Google Scholar]
- Fidler I. J., Schroit A. J. Recognition and destruction of neoplastic cells by activated macrophages: discrimination of altered self. Biochim Biophys Acta. 1988 Nov 15;948(2):151–173. doi: 10.1016/0304-419x(88)90009-1. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Gorsky L. D., Pollock J. S., Ishii K., Schmidt H. H., Heller M., Murad F. Hormone-induced biosynthesis of endothelium-derived relaxing factor/nitric oxide-like material in N1E-115 neuroblastoma cells requires calcium and calmodulin. Mol Pharmacol. 1990 Jul;38(1):7–13. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Jenkins D. C., Charles I. G., Thomsen L. L., Moss D. W., Holmes L. S., Baylis S. A., Rhodes P., Westmore K., Emson P. C., Moncada S. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonov S. A., Wells A. U., O'Connor B. J., Cole P. J., Hansell D. M., Logan-Sinclair R. B., Barnes P. J. Elevated levels of exhaled nitric oxide in bronchiectasis. Am J Respir Crit Care Med. 1995 Jun;151(6):1889–1893. doi: 10.1164/ajrccm.151.6.7767536. [DOI] [PubMed] [Google Scholar]
- Kuo H. P., Yu C. T. Alveolar macrophage subpopulations in patients with active pulmonary tuberculosis. Chest. 1993 Dec;104(6):1773–1778. doi: 10.1378/chest.104.6.1773. [DOI] [PubMed] [Google Scholar]
- Martin J. H., Edwards S. W. Changes in mechanisms of monocyte/macrophage-mediated cytotoxicity during culture. Reactive oxygen intermediates are involved in monocyte-mediated cytotoxicity, whereas reactive nitrogen intermediates are employed by macrophages in tumor cell killing. J Immunol. 1993 Apr 15;150(8 Pt 1):3478–3486. [PubMed] [Google Scholar]
- Massaro A. F., Mehta S., Lilly C. M., Kobzik L., Reilly J. J., Drazen J. M. Elevated nitric oxide concentrations in isolated lower airway gas of asthmatic subjects. Am J Respir Crit Care Med. 1996 May;153(5):1510–1514. doi: 10.1164/ajrccm.153.5.8630594. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Mordan L. J., Burnett T. S., Zhang L. X., Tom J., Cooney R. V. Inhibitors of endogenous nitrogen oxide formation block the promotion of neoplastic transformation in C3H 10T1/2 fibroblasts. Carcinogenesis. 1993 Aug;14(8):1555–1559. doi: 10.1093/carcin/14.8.1555. [DOI] [PubMed] [Google Scholar]
- Muñoz-Fernández M. A., Fernández M. A., Fresno M. Activation of human macrophages for the killing of intracellular Trypanosoma cruzi by TNF-alpha and IFN-gamma through a nitric oxide-dependent mechanism. Immunol Lett. 1992 Jun;33(1):35–40. doi: 10.1016/0165-2478(92)90090-b. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Penn I. Cancer is a complication of severe immunosuppression. Surg Gynecol Obstet. 1986 Jun;162(6):603–610. [PubMed] [Google Scholar]
- Radomski M. W., Jenkins D. C., Holmes L., Moncada S. Human colorectal adenocarcinoma cells: differential nitric oxide synthesis determines their ability to aggregate platelets. Cancer Res. 1991 Nov 15;51(22):6073–6078. [PubMed] [Google Scholar]
- Sagar S. M., Singh G., Hodson D. I., Whitton A. C. Nitric oxide and anti-cancer therapy. Cancer Treat Rev. 1995 Mar;21(2):159–181. doi: 10.1016/0305-7372(95)90025-x. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo S., Yasumoto K., Nagashima A., Nakahashi H., Sugimachi K., Nomoto K. Role of tumor-associated macrophages in lung cancer. Cancer Res. 1986 Jun;46(6):3179–3182. [PubMed] [Google Scholar]
- Thomsen L. L., Lawton F. G., Knowles R. G., Beesley J. E., Riveros-Moreno V., Moncada S. Nitric oxide synthase activity in human gynecological cancer. Cancer Res. 1994 Mar 1;54(5):1352–1354. [PubMed] [Google Scholar]
- Thomsen L. L., Miles D. W., Happerfield L., Bobrow L. G., Knowles R. G., Moncada S. Nitric oxide synthase activity in human breast cancer. Br J Cancer. 1995 Jul;72(1):41–44. doi: 10.1038/bjc.1995.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiotou V., Deliconstantinos G. Nitric oxide, peroxynitrite and nitroso-compounds formation by ultraviolet A (UVA) irradiated human squamous cell carcinoma: potential role of nitric oxide in cancer prognosis. Anticancer Res. 1995 May-Jun;15(3):931–942. [PubMed] [Google Scholar]
- Walter S., Govoni D., Bottazzi B., Mantovani A. The role of macrophages in the regulation of primary tumor growth. Pathobiology. 1991;59(4):239–242. doi: 10.1159/000163654. [DOI] [PubMed] [Google Scholar]




