Abstract
Group A streptococci have traditionally been categorized into two broad groups based on the presence or absence of serum opacity factor (OF). Recent studies show that these two groups vary in a number of properties in addition to the OF phenotype, including sequence variations in the constant region of the antiphagocytic M protein genes, the presence or absence of immunoglobulin G Fc receptor proteins, and the presence or absence of multiple M protein-like genes situated in a tandem array. The M protein genes (emm) in OF- streptococcal strains are known to be part of a regulon of virulence-related genes controlled by the trans-acting positive regulatory gene, virR, situated just upstream of emm. In OF+ strains, however, the region adjacent to virR is occupied by an M protein-related, type IIa immunoglobulin G Fc receptor gene (fcrA), and the relative position of emm has not been determined. To further define the vir regulon in OF+ streptococci, we used the polymerase chain reaction to show that fcrA49 is situated immediately upstream of emm49 in the OF+ type 49 strain CS101. This result shows for the first time the separate identity and genetic linkage of these two genes in the vir regulon of an OF+ group A streptococcal strain and confirms our previous hypothesis that emm49 exists as the central gene in a trio of emm-like genes. Additionally, using DNA hybridizations, we found considerable sequence divergence between OF- and OF+ group A streptococci in virR and in the noncoding sequences between virR and the emm or fcrA expression site. We found, however, a high degree of sequence conservation in this region within each of the two groups of strains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bak A. L., Christiansen C., Stenderup A. Bacterial genome sizes determined by DNA renaturation studies. J Gen Microbiol. 1970 Dec;64(3):377–380. doi: 10.1099/00221287-64-3-377. [DOI] [PubMed] [Google Scholar]
- Bessen D. E., Fischetti V. A. Differentiation between two biologically distinct classes of group A streptococci by limited substitutions of amino acids within the shared region of M protein-like molecules. J Exp Med. 1990 Dec 1;172(6):1757–1764. doi: 10.1084/jem.172.6.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen D. E., Fischetti V. A. Nucleotide sequences of two adjacent M or M-like protein genes of group A streptococci: different RNA transcript levels and identification of a unique immunoglobulin A-binding protein. Infect Immun. 1992 Jan;60(1):124–135. doi: 10.1128/iai.60.1.124-135.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen D., Fischetti V. A. A human IgG receptor of group A streptococci is associated with tissue site of infection and streptococcal class. J Infect Dis. 1990 Apr;161(4):747–754. doi: 10.1093/infdis/161.4.747. [DOI] [PubMed] [Google Scholar]
- Bessen D., Jones K. F., Fischetti V. A. Evidence for two distinct classes of streptococcal M protein and their relationship to rheumatic fever. J Exp Med. 1989 Jan 1;169(1):269–283. doi: 10.1084/jem.169.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparon M. G., Scott J. R. Identification of a gene that regulates expression of M protein, the major virulence determinant of group A streptococci. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8677–8681. doi: 10.1073/pnas.84.23.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C., Cleary P. P. Cloning and expression of the streptococcal C5a peptidase gene in Escherichia coli: linkage to the type 12 M protein gene. Infect Immun. 1989 Jun;57(6):1740–1745. doi: 10.1128/iai.57.6.1740-1745.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. L., Hunsaker W. R. Improved hybridization assays employing tailed oligonucleotide probes: a direct comparison with 5'-end-labeled oligonucleotide probes and nick-translated plasmid probes. Anal Biochem. 1985 Dec;151(2):211–224. doi: 10.1016/0003-2697(85)90168-x. [DOI] [PubMed] [Google Scholar]
- Frithz E., Hedén L. O., Lindahl G. Extensive sequence homology between IgA receptor and M proteins in Streptococcus pyogenes. Mol Microbiol. 1989 Aug;3(8):1111–1119. doi: 10.1111/j.1365-2958.1989.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Gomi H., Hozumi T., Hattori S., Tagawa C., Kishimoto F., Björck L. The gene sequence and some properties of protein H. A novel IgG-binding protein. J Immunol. 1990 May 15;144(10):4046–4052. [PubMed] [Google Scholar]
- Haanes-Fritz E., Kraus W., Burdett V., Dale J. B., Beachey E. H., Cleary P. Comparison of the leader sequences of four group A streptococcal M protein genes. Nucleic Acids Res. 1988 May 25;16(10):4667–4677. doi: 10.1093/nar/16.10.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
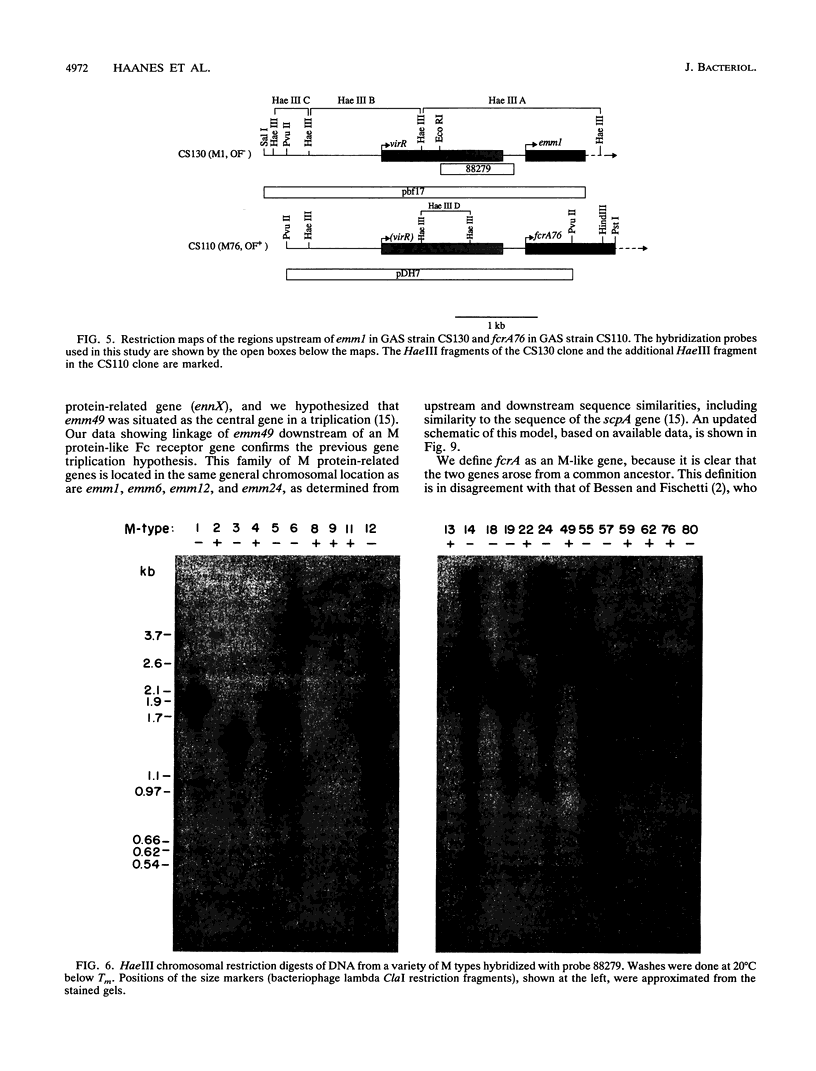
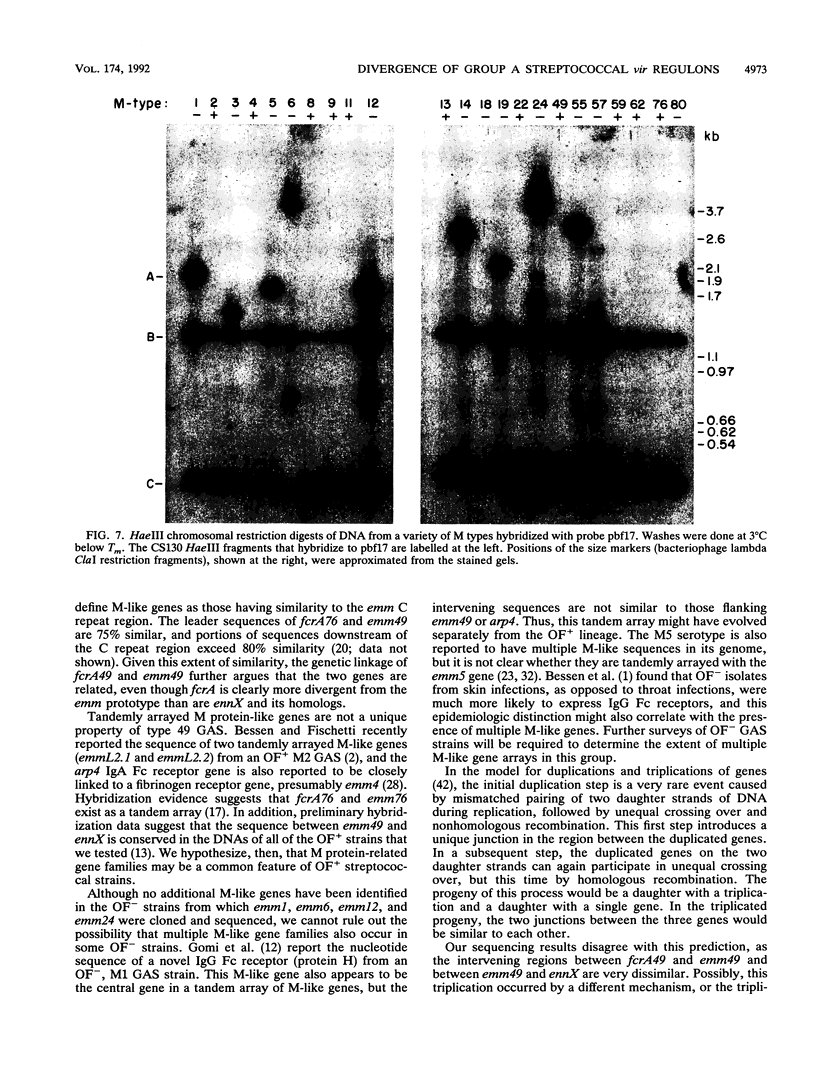
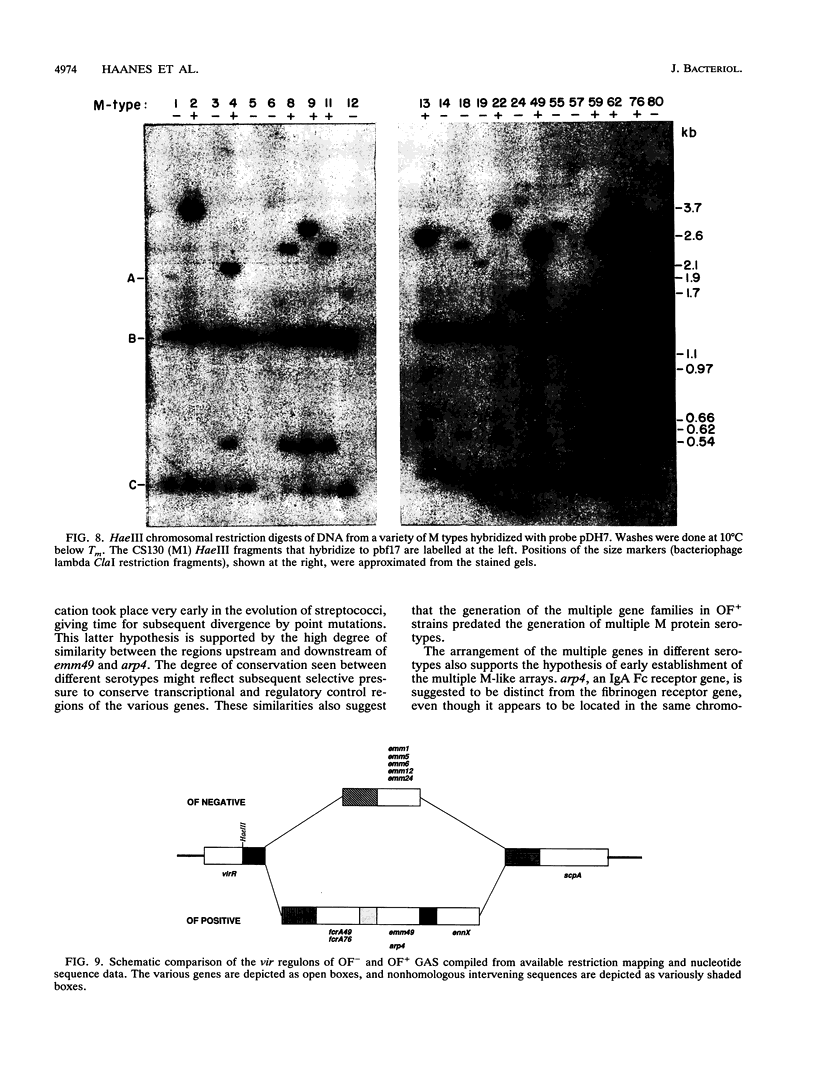
- Haanes E. J., Cleary P. P. Identification of a divergent M protein gene and an M protein-related gene family in Streptococcus pyogenes serotype 49. J Bacteriol. 1989 Dec;171(12):6397–6408. doi: 10.1128/jb.171.12.6397-6408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallas G., Widdowson J. P. The opacity factor of group-A streptococci. J Med Microbiol. 1982 Nov;15(4):451–464. doi: 10.1099/00222615-15-4-451. [DOI] [PubMed] [Google Scholar]
- Heath D. G., Boyle M. D., Cleary P. P. Isolated DNA repeat region from fcrA76, the Fc-binding protein gene from an M-type 76 strain of group A streptococci, encodes a protein with Fc-binding activity. Mol Microbiol. 1990 Dec;4(12):2071–2079. doi: 10.1111/j.1365-2958.1990.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Heath D. G., Cleary P. P. Cloning and expression of the gene for an immunoglobulin G Fc receptor protein from a group A streptococcus. Infect Immun. 1987 May;55(5):1233–1238. doi: 10.1128/iai.55.5.1233-1238.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D. G., Cleary P. P. Fc-receptor and M-protein genes of group A streptococci are products of gene duplication. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4741–4745. doi: 10.1073/pnas.86.12.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. J., Wannamaker L. W. The serum opacity reaction of Streptococcus pyogenes: general properties of the streptococcal factor and of the reaction in aged serum. J Hyg (Lond) 1968 Mar;66(1):37–47. doi: 10.1017/s0022172400040924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Complete nucleotide sequence of type 6 M protein of the group A Streptococcus. Repetitive structure and membrane anchor. J Biol Chem. 1986 Feb 5;261(4):1677–1686. [PubMed] [Google Scholar]
- Kehoe M. A., Poirier T. P., Beachey E. H., Timmis K. N. Cloning and genetic analysis of serotype 5 M protein determinant of group A streptococci: evidence for multiple copies of the M5 determinant in the Streptococcus pyogenes genome. Infect Immun. 1985 Apr;48(1):190–197. doi: 10.1128/iai.48.1.190-197.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- Lindahl G. Cell surface proteins of a group A streptococcus type M4: the IgA receptor and a receptor related to M proteins are coded for by closely linked genes. Mol Gen Genet. 1989 Apr;216(2-3):372–379. doi: 10.1007/BF00334378. [DOI] [PubMed] [Google Scholar]
- Maxted W. R. Group A streptococci: pathogenesis and immunity. Soc Appl Bacteriol Symp Ser. 1978;7:107–125. [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller L., Gray L., Beachey E., Kehoe M. Antigenic variation among group A streptococcal M proteins. Nucleotide sequence of the serotype 5 M protein gene and its relationship with genes encoding types 6 and 24 M proteins. J Biol Chem. 1988 Apr 25;263(12):5668–5673. [PubMed] [Google Scholar]
- Mouw A. R., Beachey E. H., Burdett V. Molecular evolution of streptococcal M protein: cloning and nucleotide sequence of the type 24 M protein gene and relation to other genes of Streptococcus pyogenes. J Bacteriol. 1988 Feb;170(2):676–684. doi: 10.1128/jb.170.2.676-684.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Musser J. M., Hauser A. R., Kim M. H., Schlievert P. M., Nelson K., Selander R. K. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2668–2672. doi: 10.1073/pnas.88.7.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Casal J., Caparon M. G., Scott J. R. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J Bacteriol. 1991 Apr;173(8):2617–2624. doi: 10.1128/jb.173.8.2617-2624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. C., Spanier J. G., Jones S. J., Simpson W. J., Cleary P. P. Streptococcus pyogenes type 12 M protein gene regulation by upstream sequences. J Bacteriol. 1987 Dec;169(12):5633–5640. doi: 10.1128/jb.169.12.5633-5640.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R., Pulliam W. M., Hollingshead S. K., Fischetti V. A. Relationship of M protein genes in group A streptococci. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1822–1826. doi: 10.1073/pnas.82.6.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. J., LaPenta D., Chen C., Cleary P. P. Coregulation of type 12 M protein and streptococcal C5a peptidase genes in group A streptococci: evidence for a virulence regulon controlled by the virR locus. J Bacteriol. 1990 Feb;172(2):696–700. doi: 10.1128/jb.172.2.696-700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. P. Evolution of repeated DNA sequences by unequal crossover. Science. 1976 Feb 13;191(4227):528–535. doi: 10.1126/science.1251186. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Top F. H., Jr, Wannamaker L. W. The serum opacity reaction of Streptococcus pyogenes. The demonstration of multiple, strain-specific lipoproteinase antigens. J Exp Med. 1968 May 1;127(5):1013–1034. doi: 10.1084/jem.127.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Top F. H., Jr, Wannamaker L. W. The serum opacity reaction of Streptococcus pyogenes: frequency of production of streptococcal lipoproteinase by strains of different serological types and the relationship of M protein production. J Hyg (Lond) 1968 Mar;66(1):49–58. doi: 10.1017/s0022172400040936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler D. E., Nelson R. D., Cleary P. P. Human neutrophil chemotactic response to group A streptococci: bacteria-mediated interference with complement-derived chemotactic factors. Infect Immun. 1983 Jan;39(1):239–246. doi: 10.1128/iai.39.1.239-246.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]