Abstract
A proteolysis-inducing factor (PIF) isolated from a cachexia-inducing murine tumour (MAC16) produced a decrease in body weight (1.6 g, P < or = 0.01 compared with control subjects) within 24 h after i.v. administration to non-tumour-bearing mice. Weight loss was associated with significant decreases in the weight of the spleen and soleus and gastrocnemius muscles, with no effect on the weight of the heart or kidney and with an increase in weight of the liver. Protein degradation in isolated soleus muscle was significantly increased in mice bearing the MAC16 tumour. To define which proteolytic pathways contribute to this increase, soleus muscles from mice bearing the MAC16 tumour and non-tumour-bearing animals administered PIF were incubated under conditions that modify different proteolytic systems. In mice bearing the MAC16 tumour, there were increases in both cathepsin B and L, and the Ca2+-dependent lysosomal and ATP-dependent pathways were found to contribute to the increased proteolysis; whereas, in PIF-injected animals, there was activation only of the ATP-dependent pathway. Further studies in mice bearing the MAC16 tumour have provided evidence for increased levels of ubiquitin-conjugated proteins and increased mRNA levels for the 14 kDa ubiquitin carrier protein E2 and the C9 proteasome subunit in gastrocnemius muscle, suggesting activation of the ATP-ubiquitin-dependent proteolytic pathway. A monoclonal antibody to PIF attenuated the enhanced protein degradation in soleus muscle from mice bearing the MAC16 tumour, confirming that PIF is responsible for the loss of skeletal muscle in cachectic mice.
Full text
PDF


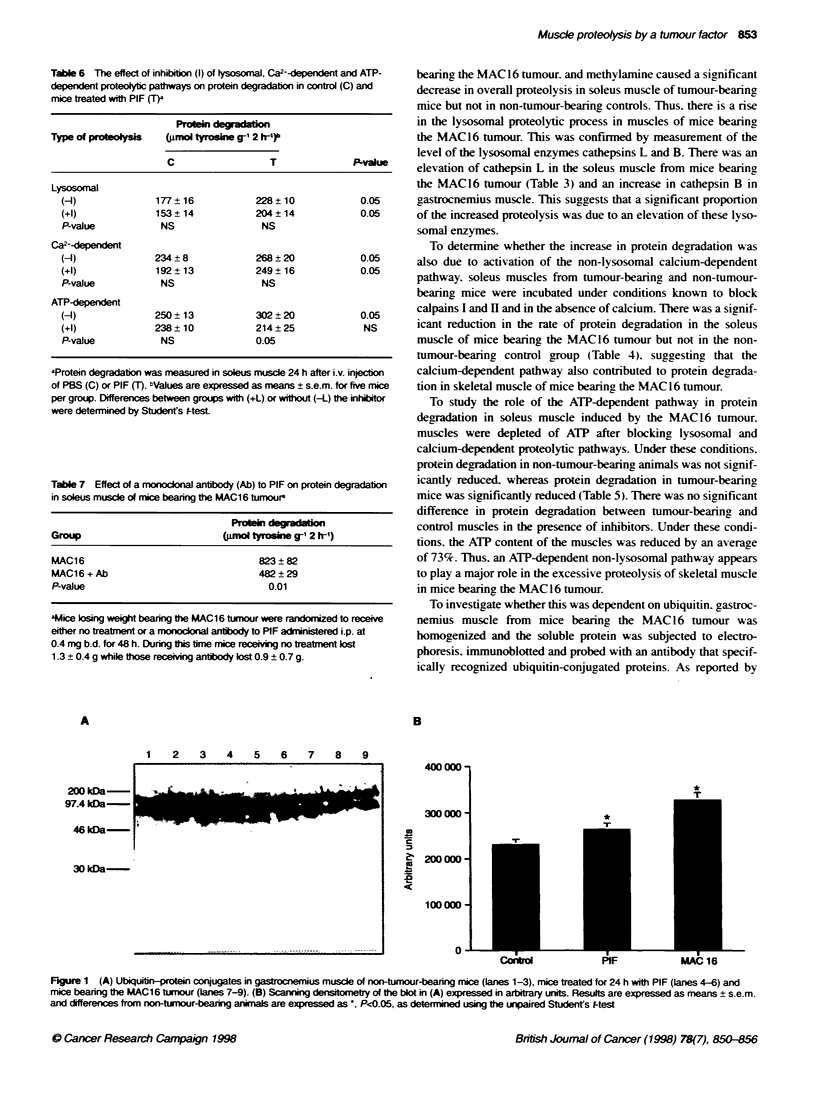
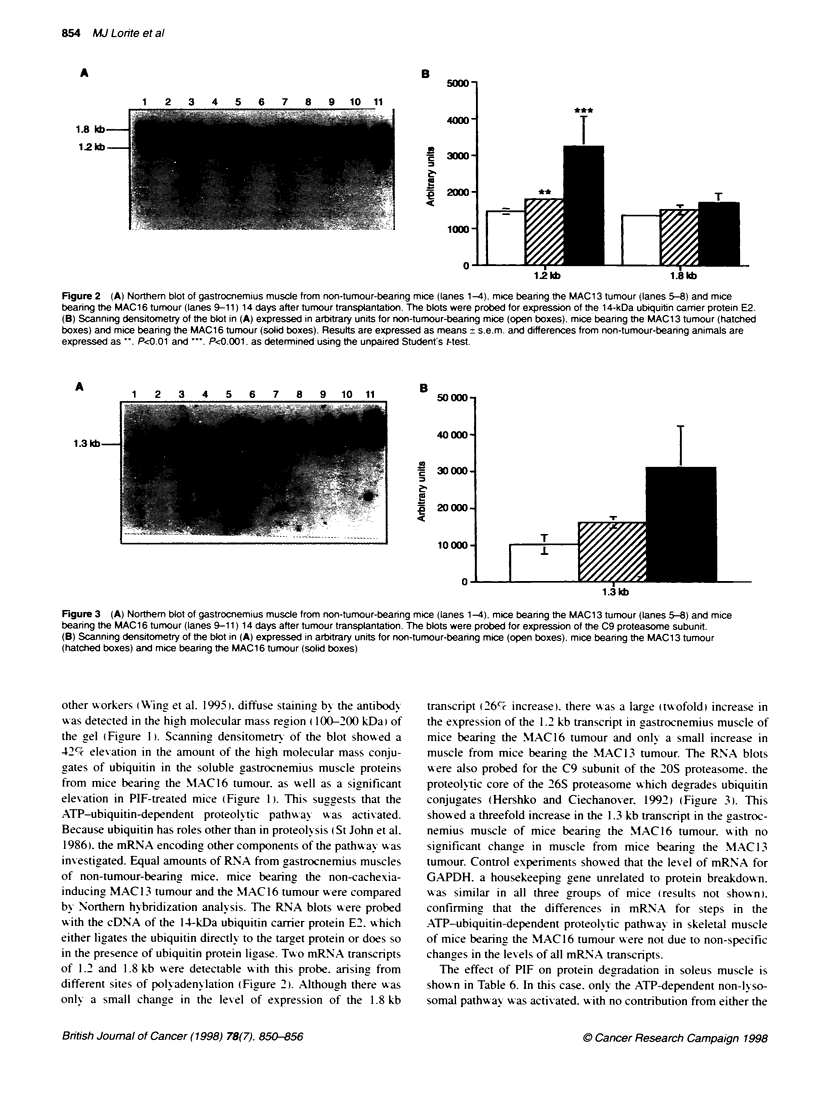


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baracos V. E., DeVivo C., Hoyle D. H., Goldberg A. L. Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am J Physiol. 1995 May;268(5 Pt 1):E996–1006. doi: 10.1152/ajpendo.1995.268.5.E996. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S. A., Tisdale M. J. Production of lipolytic and proteolytic factors by a murine tumor-producing cachexia in the host. Cancer Res. 1987 Nov 15;47(22):5919–5923. [PubMed] [Google Scholar]
- Bird J. W., Carter J. H., Triemer R. E., Brooks R. M., Spanier A. M. Proteinases in cardiac and skeletal muscle. Fed Proc. 1980 Jan;39(1):20–25. [PubMed] [Google Scholar]
- Carmichael M. J., Clague M. B., Keir M. J., Johnston I. D. Whole body protein turnover, synthesis and breakdown in patients with colorectal carcinoma. Br J Surg. 1980 Oct;67(10):736–739. doi: 10.1002/bjs.1800671015. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ebisui C., Tsujinaka T., Morimoto T., Kan K., Iijima S., Yano M., Kominami E., Tanaka K., Monden M. Interleukin-6 induces proteolysis by activating intracellular proteases (cathepsins B and L, proteasome) in C2C12 myotubes. Clin Sci (Lond) 1995 Oct;89(4):431–439. doi: 10.1042/cs0890431. [DOI] [PubMed] [Google Scholar]
- Fagan J. M., Waxman L. A novel ATP-requiring protease from skeletal muscle that hydrolyzes non-ubiquitinated proteins. J Biol Chem. 1989 Oct 25;264(30):17868–17872. [PubMed] [Google Scholar]
- Fagan J. M., Waxman L., Goldberg A. L. Skeletal muscle and liver contain a soluble ATP + ubiquitin-dependent proteolytic system. Biochem J. 1987 Apr 15;243(2):335–343. doi: 10.1042/bj2430335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- Kumatori A., Tanaka K., Tamura T., Fujiwara T., Ichihara A., Tokunaga F., Onikura A., Iwanaga S. cDNA cloning and sequencing of component C9 of proteasomes from rat hepatoma cells. FEBS Lett. 1990 May 21;264(2):279–282. doi: 10.1016/0014-5793(90)80267-m. [DOI] [PubMed] [Google Scholar]
- Llovera M., García-Martínez C., Agell N., López-Soriano F. J., Argilés J. M. TNF can directly induce the expression of ubiquitin-dependent proteolytic system in rat soleus muscles. Biochem Biophys Res Commun. 1997 Jan 13;230(2):238–241. doi: 10.1006/bbrc.1996.5827. [DOI] [PubMed] [Google Scholar]
- Llovera M., García-Martínez C., Agell N., Marzábal M., López-Soriano F. J., Argilés J. M. Ubiquitin gene expression is increased in skeletal muscle of tumour-bearing rats. FEBS Lett. 1994 Feb 7;338(3):311–318. doi: 10.1016/0014-5793(94)80290-4. [DOI] [PubMed] [Google Scholar]
- Lorite M. J., Cariuk P., Tisdale M. J. Induction of muscle protein degradation by a tumour factor. Br J Cancer. 1997;76(8):1035–1040. doi: 10.1038/bjc.1997.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholm K., Holm G., Scherstén T. Insulin resistance in patients with cancer. Cancer Res. 1978 Dec;38(12):4665–4670. [PubMed] [Google Scholar]
- Matthys P., Billiau A. Cytokines and cachexia. Nutrition. 1997 Sep;13(9):763–770. doi: 10.1016/s0899-9007(97)00185-8. [DOI] [PubMed] [Google Scholar]
- Medina R., Wing S. S., Goldberg A. L. Increase in levels of polyubiquitin and proteasome mRNA in skeletal muscle during starvation and denervation atrophy. Biochem J. 1995 May 1;307(Pt 3):631–637. doi: 10.1042/bj3070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville S., McNurlan M. A., Calder A. G., Garlick P. J. Increased protein turnover despite normal energy metabolism and responses to feeding in patients with lung cancer. Cancer Res. 1990 Feb 15;50(4):1125–1131. [PubMed] [Google Scholar]
- Mitch W. E., Medina R., Grieber S., May R. C., England B. K., Price S. R., Bailey J. L., Goldberg A. L. Metabolic acidosis stimulates muscle protein degradation by activating the adenosine triphosphate-dependent pathway involving ubiquitin and proteasomes. J Clin Invest. 1994 May;93(5):2127–2133. doi: 10.1172/JCI117208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan H. D., Mahony S. M., Ross J. A., Tisdale M. J. Weight loss in a murine cachexia model is not associated with the cytokines tumour necrosis factor-alpha or interleukin-6. Cancer Lett. 1992 Aug 31;65(3):239–243. doi: 10.1016/0304-3835(92)90238-q. [DOI] [PubMed] [Google Scholar]
- Nixon D. W., Heymsfield S. B., Cohen A. E., Kutner M. H., Ansley J., Lawson D. H., Rudman D. Protein-calorie undernutrition in hospitalized cancer patients. Am J Med. 1980 May;68(5):683–690. doi: 10.1016/0002-9343(80)90254-5. [DOI] [PubMed] [Google Scholar]
- Smith K. L., Tisdale M. J. Mechanism of muscle protein degradation in cancer cachexia. Br J Cancer. 1993 Aug;68(2):314–318. doi: 10.1038/bjc.1993.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John T., Gallatin W. M., Siegelman M., Smith H. T., Fried V. A., Weissman I. L. Expression cloning of a lymphocyte homing receptor cDNA: ubiquitin is the reactive species. Science. 1986 Feb 21;231(4740):845–850. doi: 10.1126/science.3003914. [DOI] [PubMed] [Google Scholar]
- Temparis S., Asensi M., Taillandier D., Aurousseau E., Larbaud D., Obled A., Béchet D., Ferrara M., Estrela J. M., Attaix D. Increased ATP-ubiquitin-dependent proteolysis in skeletal muscles of tumor-bearing rats. Cancer Res. 1994 Nov 1;54(21):5568–5573. [PubMed] [Google Scholar]
- Tiao G., Fagan J. M., Samuels N., James J. H., Hudson K., Lieberman M., Fischer J. E., Hasselgren P. O. Sepsis stimulates nonlysosomal, energy-dependent proteolysis and increases ubiquitin mRNA levels in rat skeletal muscle. J Clin Invest. 1994 Dec;94(6):2255–2264. doi: 10.1172/JCI117588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov P. T., Deacon M., Tisdale M. J. Structural analysis of a tumor-produced sulfated glycoprotein capable of initiating muscle protein degradation. J Biol Chem. 1997 May 9;272(19):12279–12288. doi: 10.1074/jbc.272.19.12279. [DOI] [PubMed] [Google Scholar]
- Todorov P. T., McDevitt T. M., Cariuk P., Coles B., Deacon M., Tisdale M. J. Induction of muscle protein degradation and weight loss by a tumor product. Cancer Res. 1996 Mar 15;56(6):1256–1261. [PubMed] [Google Scholar]
- Todorov P., Cariuk P., McDevitt T., Coles B., Fearon K., Tisdale M. Characterization of a cancer cachectic factor. Nature. 1996 Feb 22;379(6567):739–742. doi: 10.1038/379739a0. [DOI] [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]
- Waxman L. Calcium-activated proteases in mammalian tissues. Methods Enzymol. 1981;80(Pt 100):664–680. doi: 10.1016/s0076-6879(81)80051-1. [DOI] [PubMed] [Google Scholar]
- Wing S. S., Haas A. L., Goldberg A. L. Increase in ubiquitin-protein conjugates concomitant with the increase in proteolysis in rat skeletal muscle during starvation and atrophy denervation. Biochem J. 1995 May 1;307(Pt 3):639–645. doi: 10.1042/bj3070639. [DOI] [PMC free article] [PubMed] [Google Scholar]





