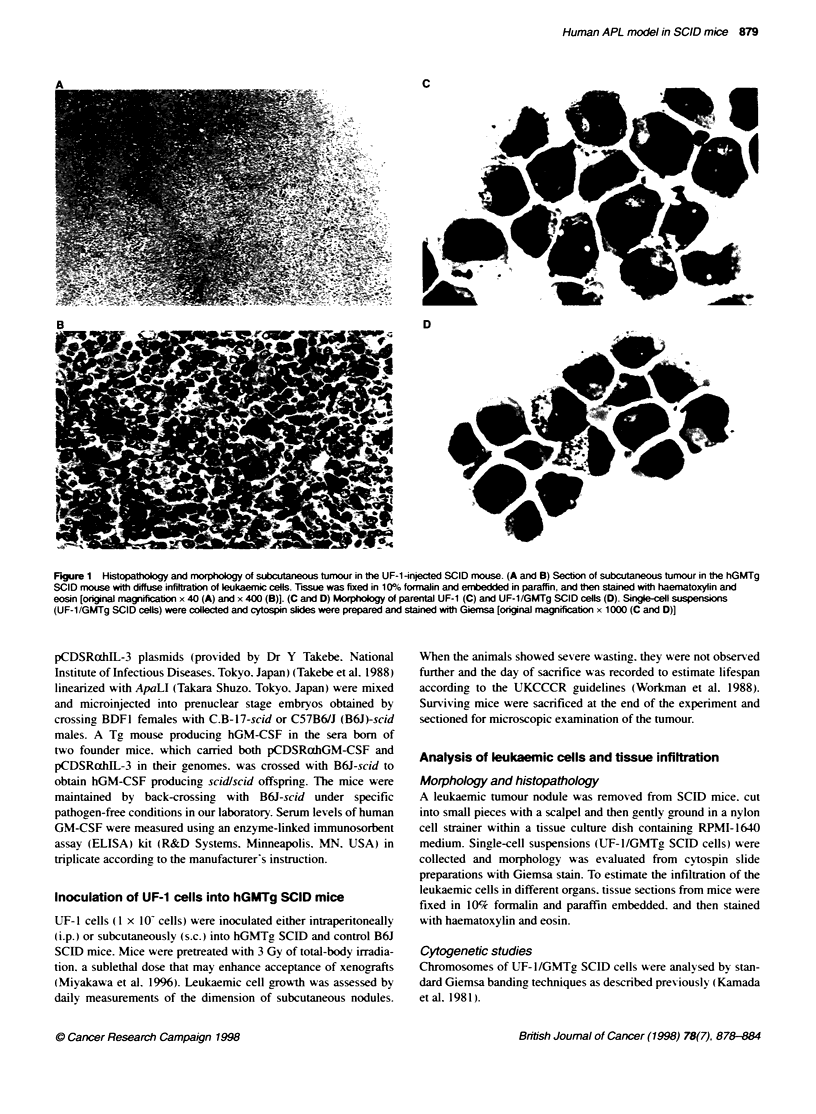
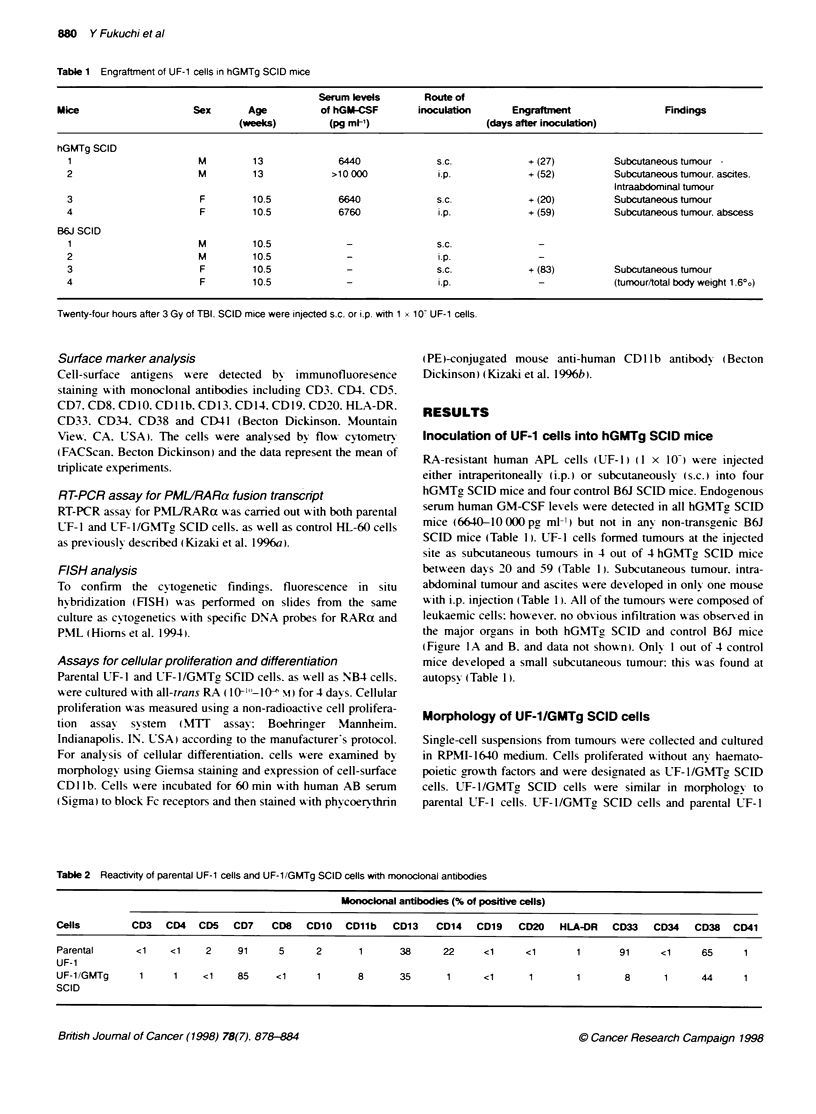
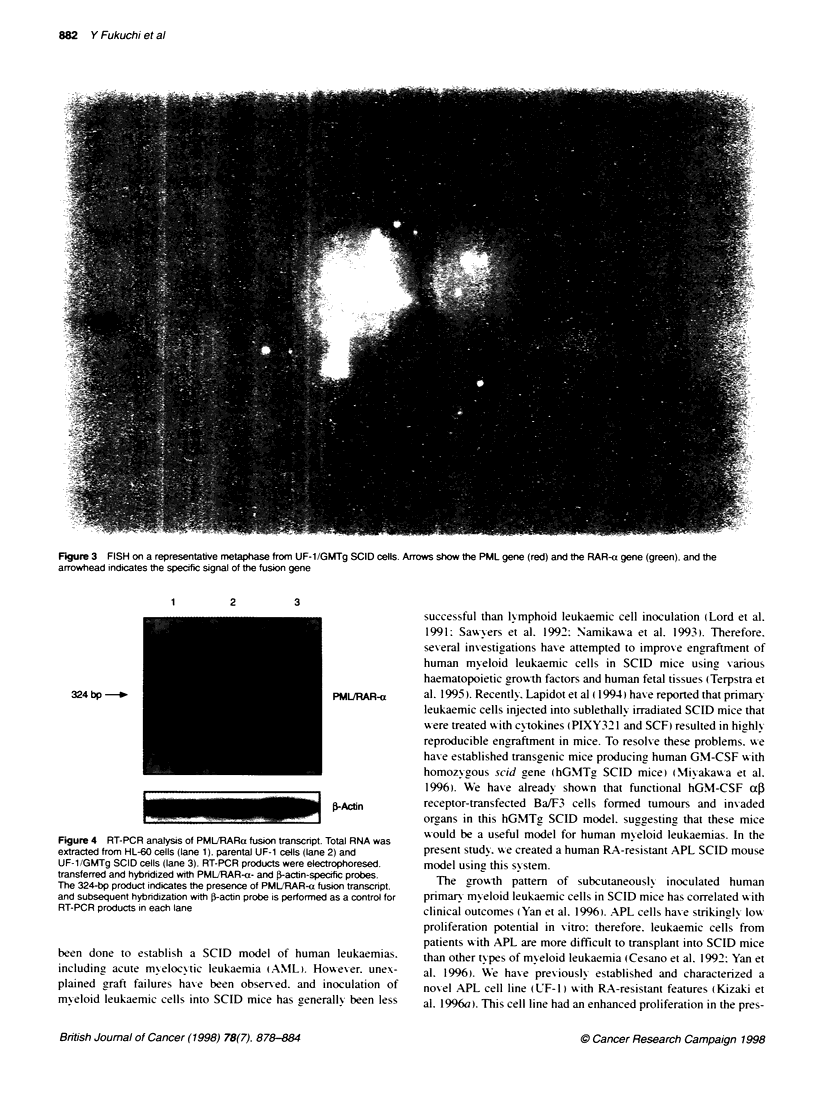
Abstract
To understand the mechanisms and identify novel approaches to overcoming retinoic acid (RA) resistance in acute promyelocytic leukaemia (APL), we established the first human RA-resistant APL model in severe combined immunodeficiency (SCID) mice. UF-1 cells, an RA-resistant APL cell line established in our laboratory, were transplanted into human granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing SCID (hGMTg SCID) mice and inoculated cells formed subcutaneous tumours in all hGMTg SCID mice, but not in the non-transgenic control SCID mice. Single-cell suspensions (UF-1/GMTg SCID cells) were similar in morphological, immunological, cytogenetic and molecular genetic features to parental UF-1 cells. All-trans RA did not change the morphological features of cells or their expression of CD11b. RA did not alter the growth curve of cells as determined by MTT assay, suggesting that UF-1/GMTg SCID cells are resistant to RA. These results demonstrate that this is the first RA-resistant APL animal model that may be useful for investigating the biology of this myeloid leukaemia in vivo, as well as for evaluating novel therapeutic approaches including patients with RA-resistant APL.
Full text
PDF

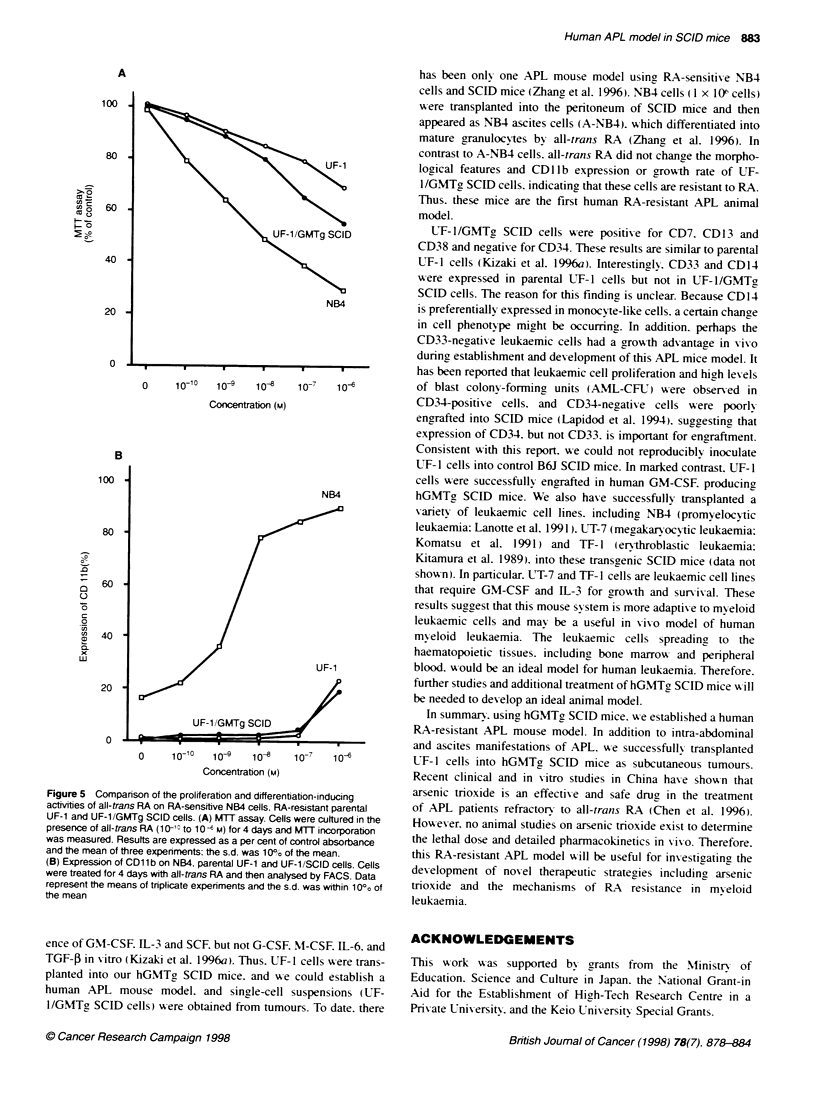

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cesano A., Hoxie J. A., Lange B., Nowell P. C., Bishop J., Santoli D. The severe combined immunodeficient (SCID) mouse as a model for human myeloid leukemias. Oncogene. 1992 May;7(5):827–836. [PubMed] [Google Scholar]
- Chen G. Q., Zhu J., Shi X. G., Ni J. H., Zhong H. J., Si G. Y., Jin X. L., Tang W., Li X. S., Xong S. M. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996 Aug 1;88(3):1052–1061. [PubMed] [Google Scholar]
- De Lord C., Clutterbuck R., Titley J., Ormerod M., Gordon-Smith T., Millar J., Powles R. Growth of primary human acute leukemia in severe combined immunodeficient mice. Exp Hematol. 1991 Oct;19(9):991–993. [PubMed] [Google Scholar]
- Huang M. E., Ye Y. C., Chen S. R., Chai J. R., Lu J. X., Zhoa L., Gu L. J., Wang Z. Y. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988 Aug;72(2):567–572. [PubMed] [Google Scholar]
- Kamada N., Dohy H., Okada K., Oguma N., Kuramoto A., Tanaka K., Uchino H. In vivo and in vitro activity of neutrophil alkaline phosphatase in acute myelocytic leukemia with 8;21 translocation. Blood. 1981 Dec;58(6):1213–1217. [PubMed] [Google Scholar]
- Kanamaru A., Takemoto Y., Tanimoto M., Murakami H., Asou N., Kobayashi T., Kuriyama K., Ohmoto E., Sakamaki H., Tsubaki K. All-trans retinoic acid for the treatment of newly diagnosed acute promyelocytic leukemia. Japan Adult Leukemia Study Group. Blood. 1995 Mar 1;85(5):1202–1206. [PubMed] [Google Scholar]
- Kitamura T., Tange T., Terasawa T., Chiba S., Kuwaki T., Miyagawa K., Piao Y. F., Miyazono K., Urabe A., Takaku F. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol. 1989 Aug;140(2):323–334. doi: 10.1002/jcp.1041400219. [DOI] [PubMed] [Google Scholar]
- Kizaki M., Matsushita H., Takayama N., Muto A., Ueno H., Awaya N., Kawai Y., Asou H., Kamada N., Ikeda Y. Establishment and characterization of a novel acute promyelocytic leukemia cell line (UF-1) with retinoic acid-resistant features. Blood. 1996 Sep 1;88(5):1824–1833. [PubMed] [Google Scholar]
- Kizaki M., Ueno H., Yamazoe Y., Shimada M., Takayama N., Muto A., Matsushita H., Nakajima H., Morikawa M., Koeffler H. P. Mechanisms of retinoid resistance in leukemic cells: possible role of cytochrome P450 and P-glycoprotein. Blood. 1996 Jan 15;87(2):725–733. [PubMed] [Google Scholar]
- Komatsu N., Nakauchi H., Miwa A., Ishihara T., Eguchi M., Moroi M., Okada M., Sato Y., Wada H., Yawata Y. Establishment and characterization of a human leukemic cell line with megakaryocytic features: dependency on granulocyte-macrophage colony-stimulating factor, interleukin 3, or erythropoietin for growth and survival. Cancer Res. 1991 Jan 1;51(1):341–348. [PubMed] [Google Scholar]
- Lanotte M., Martin-Thouvenin V., Najman S., Balerini P., Valensi F., Berger R. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3). Blood. 1991 Mar 1;77(5):1080–1086. [PubMed] [Google Scholar]
- Lapidot T., Sirard C., Vormoor J., Murdoch B., Hoang T., Caceres-Cortes J., Minden M., Paterson B., Caligiuri M. A., Dick J. E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994 Feb 17;367(6464):645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Miyakawa Y., Fukuchi Y., Ito M., Kobayashi K., Kuramochi T., Ikeda Y., Takebe Y., Tanaka T., Miyasaka M., Nakahata T. Establishment of human granulocyte-macrophage colony stimulating factor producing transgenic SCID mice. Br J Haematol. 1996 Dec;95(3):437–442. doi: 10.1046/j.1365-2141.1996.8012423.x. [DOI] [PubMed] [Google Scholar]
- Namikawa R., Ueda R., Kyoizumi S. Growth of human myeloid leukemias in the human marrow environment of SCID-hu mice. Blood. 1993 Oct 15;82(8):2526–2536. [PubMed] [Google Scholar]
- Sawyers C. L., Gishizky M. L., Quan S., Golde D. W., Witte O. N. Propagation of human blastic myeloid leukemias in the SCID mouse. Blood. 1992 Apr 15;79(8):2089–2098. [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra W., Prins A., Visser T., Wognum B., Wagemaker G., Löwenberg B., Wielenga J. Conditions for engraftment of human acute myeloid leukemia (AML) in SCID mice. Leukemia. 1995 Sep;9(9):1573–1577. [PubMed] [Google Scholar]
- Uckun F. M. Severe combined immunodeficient mouse models of human leukemia. Blood. 1996 Aug 15;88(4):1135–1146. [PubMed] [Google Scholar]
- Warrell R. P., Jr, Frankel S. R., Miller W. H., Jr, Scheinberg D. A., Itri L. M., Hittelman W. N., Vyas R., Andreeff M., Tafuri A., Jakubowski A. Differentiation therapy of acute promyelocytic leukemia with tretinoin (all-trans-retinoic acid). N Engl J Med. 1991 May 16;324(20):1385–1393. doi: 10.1056/NEJM199105163242002. [DOI] [PubMed] [Google Scholar]
- Warrell R. P., Jr, de Thé H., Wang Z. Y., Degos L. Acute promyelocytic leukemia. N Engl J Med. 1993 Jul 15;329(3):177–189. doi: 10.1056/NEJM199307153290307. [DOI] [PubMed] [Google Scholar]
- Yan Y., Salomon O., McGuirk J., Dennig D., Fernandez J., Jagiello C., Nguyen H., Collins N., Steinherz P., O'Reilly R. J. Growth pattern and clinical correlation of subcutaneously inoculated human primary acute leukemias in severe combined immunodeficiency mice. Blood. 1996 Oct 15;88(8):3137–3146. [PubMed] [Google Scholar]
- Zhang S. Y., Zhu J., Chen G. Q., Du X. X., Lu L. J., Zhang Z., Zhong H. J., Chen H. R., Wang Z. Y., Berger R. Establishment of a human acute promyelocytic leukemia-ascites model in SCID mice. Blood. 1996 Apr 15;87(8):3404–3409. [PubMed] [Google Scholar]