Abstract
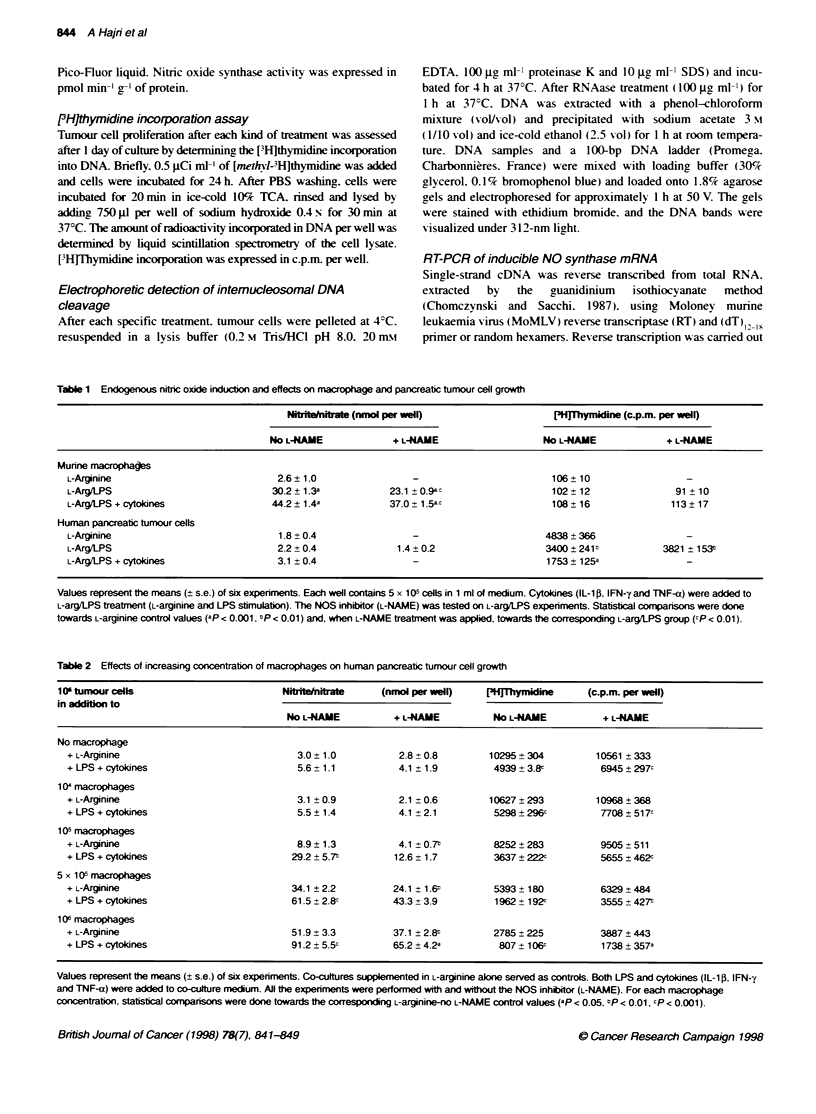
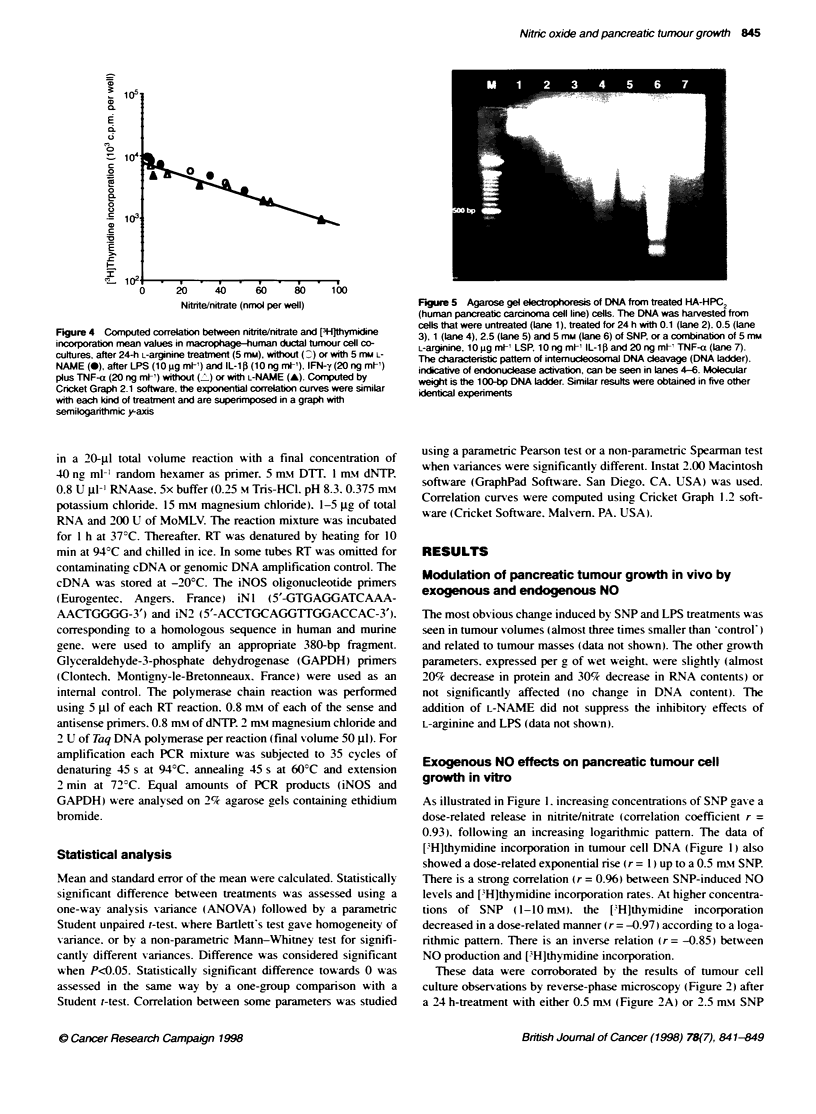
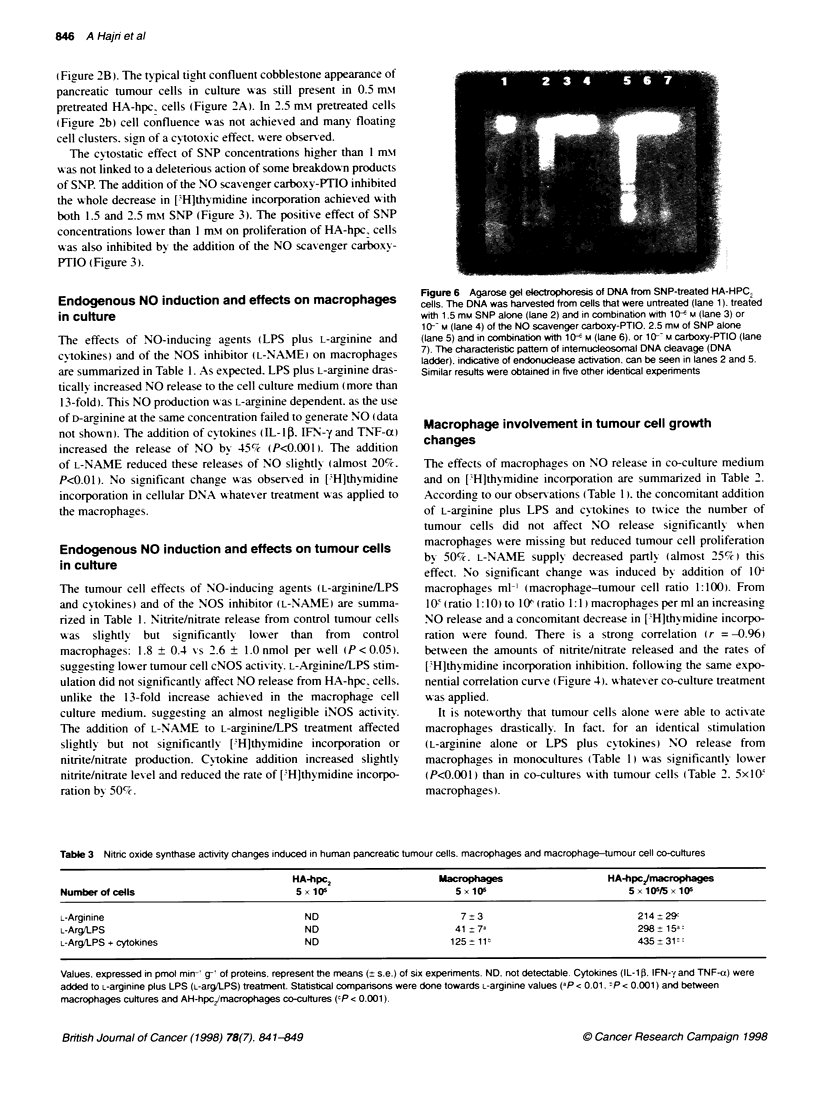
Nitric oxide (NO), an endogenous free radical, has been implicated in a wide range of biological functions. NO is generated enzymatically from the terminal guanidinonitrogen of L-arginine by nitric oxide synthase (NOS). Despite intensive investigations, the role of NO--either as the primary product of the L-arginine/NOS pathway or provided from the NO donor sodium nitroprusside (SNP)--in carcinogenesis and tumour cell growth remains unclear and controversial. The objective of this study was to examine the growth effects of NO on a ductal pancreatic adenocarcinoma in the rat and on a human pancreatic tumour cell line (HA-hpc2). In vivo, both SNP and endogenous induction of NO by endotoxins [lipopolysaccharide (LPS)] plus L-arginine significantly reduced the tumour growth. To investigate the mechanisms of NO anti-tumour growth action, the effects of either the SNP or L-arginine/NOS pathway were analysed on the HA-hpc2 cell line. Nitrite/nitrate production, NOS activity and iNOS expression [assessed by reverse transcription-polymerase chain reaction (RT-PCR)] were tested and related to growth (assessed by [3H]thymidine incorporation assay) and apoptosis (assessed by internucleosomal DNA cleavage). SNP exerted a dual effect on tumour cells: stimulation of the proliferation up to 1 mM and inhibition at higher concentrations. These effects were related to NO production. Both proliferative and cytostatic responses were inhibited by NO scavenger 2-phenyl-4,4,5,5-tetramethyl-hemidazoline-1-oxyl3-oxide (carboxy-PTIO). The marked apoptotic DNA fragmentation induced by SNP was also abolished by PTIO association. Unlike macrophages, the human pancreatic tumour cells did not seem to express intrinsically the L-arginine/NOS pathway. Macrophages were activated by HA-hpc2 cells as well as by LPS plus cytokines [interleukin (IL)-1beta plus tumour necrosis factor (TNF)-alpha and interferon (IFN)-gamma]. In HA-hpc2/macrophage co-cultures, NOS activity and inducible NOS (iNOS) transcription were stimulated, whereas an antiproliferative response was observed. These effects were related to both macrophage amount and NO production. Addition of LPS plus cytokines to co-cultures doubled iNOS activity, nitrite/nitrate production and tumoricidal effect. These data suggest the involvement of NO in pancreatic tumour growth and support the fact that generation of high levels of NO with potential production of endogenous reactive nitrogen intermediates may contribute to induction of apoptosis and tumour growth inhibition.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Shimosegawa T., Satoh A., Abe R., Kikuchi Y., Koizumi M., Toyota T. Nitric oxide modulates pancreatic edema formation in rat caerulein-induced pancreatitis. J Gastroenterol. 1995 Oct;30(5):636–642. doi: 10.1007/BF02367791. [DOI] [PubMed] [Google Scholar]
- Adler H., Adler B., Peveri P., Werner E. R., Wachter H., Peterhans E., Jungi T. W. Differential regulation of inducible nitric oxide synthase production in bovine and caprine macrophages. J Infect Dis. 1996 Apr;173(4):971–978. doi: 10.1093/infdis/173.4.971. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. J., Xie Q. W., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Nathan C. Calmodulin is a subunit of nitric oxide synthase from macrophages. J Exp Med. 1992 Aug 1;176(2):599–604. doi: 10.1084/jem.176.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Esumi H., Tannenbaum S. R. U.S.-Japan Cooperative Cancer Research Program: seminar on nitric oxide synthase and carcinogenesis. Cancer Res. 1994 Jan 1;54(1):297–301. [PubMed] [Google Scholar]
- Gabbott P. L., Bacon S. J. Histochemical localization of NADPH-dependent diaphorase (nitric oxide synthase) activity in vascular endothelial cells in the rat brain. Neuroscience. 1993 Nov;57(1):79–95. doi: 10.1016/0306-4522(93)90113-t. [DOI] [PubMed] [Google Scholar]
- Guo F. H., De Raeve H. R., Rice T. W., Stuehr D. J., Thunnissen F. B., Erzurum S. C. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7809–7813. doi: 10.1073/pnas.92.17.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle E. S., Linn S. Formation, prevention, and repair of DNA damage by iron/hydrogen peroxide. J Biol Chem. 1997 Aug 1;272(31):19095–19098. doi: 10.1074/jbc.272.31.19095. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr Synthesis of nitric oxide from L-arginine: a recently discovered pathway induced by cytokines with antitumour and antimicrobial activity. Res Immunol. 1991 Sep;142(7):565–598. doi: 10.1016/0923-2494(91)90103-p. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Westenfelder C., Taintor R., Vavrin Z., Kablitz C., Baranowski R. L., Ward J. H., Menlove R. L., McMurry M. P., Kushner J. P. Evidence for cytokine-inducible nitric oxide synthesis from L-arginine in patients receiving interleukin-2 therapy. J Clin Invest. 1992 Mar;89(3):867–877. doi: 10.1172/JCI115666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibuki Y., Goto R. Enhancement of NO production from resident peritoneal macrophages by in vitro gamma-irradiation and its relationship to reactive oxygen intermediates. Free Radic Biol Med. 1997;22(6):1029–1035. doi: 10.1016/s0891-5849(96)00500-x. [DOI] [PubMed] [Google Scholar]
- Jenkins D. C., Charles I. G., Baylis S. A., Lelchuk R., Radomski M. W., Moncada S. Human colon cancer cell lines show a diverse pattern of nitric oxide synthase gene expression and nitric oxide generation. Br J Cancer. 1994 Nov;70(5):847–849. doi: 10.1038/bjc.1994.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins D. C., Charles I. G., Thomsen L. L., Moss D. W., Holmes L. S., Baylis S. A., Rhodes P., Westmore K., Emson P. C., Moncada S. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laskin D. L., Rodriguez del Valle M., Heck D. E., Hwang S. M., Ohnishi S. T., Durham S. K., Goller N. L., Laskin J. D. Hepatic nitric oxide production following acute endotoxemia in rats is mediated by increased inducible nitric oxide synthase gene expression. Hepatology. 1995 Jul;22(1):223–234. [PubMed] [Google Scholar]
- Lepoivre M., Boudbid H., Petit J. F. Antiproliferative activity of gamma-interferon combined with lipopolysaccharide on murine adenocarcinoma: dependence on an L-arginine metabolism with production of nitrite and citrulline. Cancer Res. 1989 Apr 15;49(8):1970–1976. [PubMed] [Google Scholar]
- Lepoivre M., Chenais B., Yapo A., Lemaire G., Thelander L., Tenu J. P. Alterations of ribonucleotide reductase activity following induction of the nitrite-generating pathway in adenocarcinoma cells. J Biol Chem. 1990 Aug 25;265(24):14143–14149. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nguyen T., Brunson D., Crespi C. L., Penman B. W., Wishnok J. S., Tannenbaum S. R. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3030–3034. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pettengill O. S., Faris R. A., Bell R. H., Jr, Kuhlmann E. T., Longnecker D. S. Derivation of ductlike cell lines from a transplantable acinar cell carcinoma of the rat pancreas. Am J Pathol. 1993 Jul;143(1):292–303. [PMC free article] [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Synthesis of nitrite and nitrate in murine macrophage cell lines. Cancer Res. 1987 Nov 1;47(21):5590–5594. [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi N., Pickett C. B., Griffith O. W. Oxy radicals and antioxidative responses in cancer: 12th Sapporo Cancer Seminar. Cancer Res. 1993 Jul 1;53(13):3207–3210. [PubMed] [Google Scholar]
- Thomas C., Nijenhuis A. M., Dontje B., Daemen T., Scherphof G. L. Tumoricidal response of liver macrophages isolated from rats bearing liver metastases of colon adenocarcinoma. J Leukoc Biol. 1995 Apr;57(4):617–623. doi: 10.1002/jlb.57.4.617. [DOI] [PubMed] [Google Scholar]
- Weinberg J. B., Chapman H. A., Jr, Hibbs J. B., Jr Characterization of the effects of endotoxin on macrophage tumor cell killing. J Immunol. 1978 Jul;121(1):72–80. [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]