Abstract
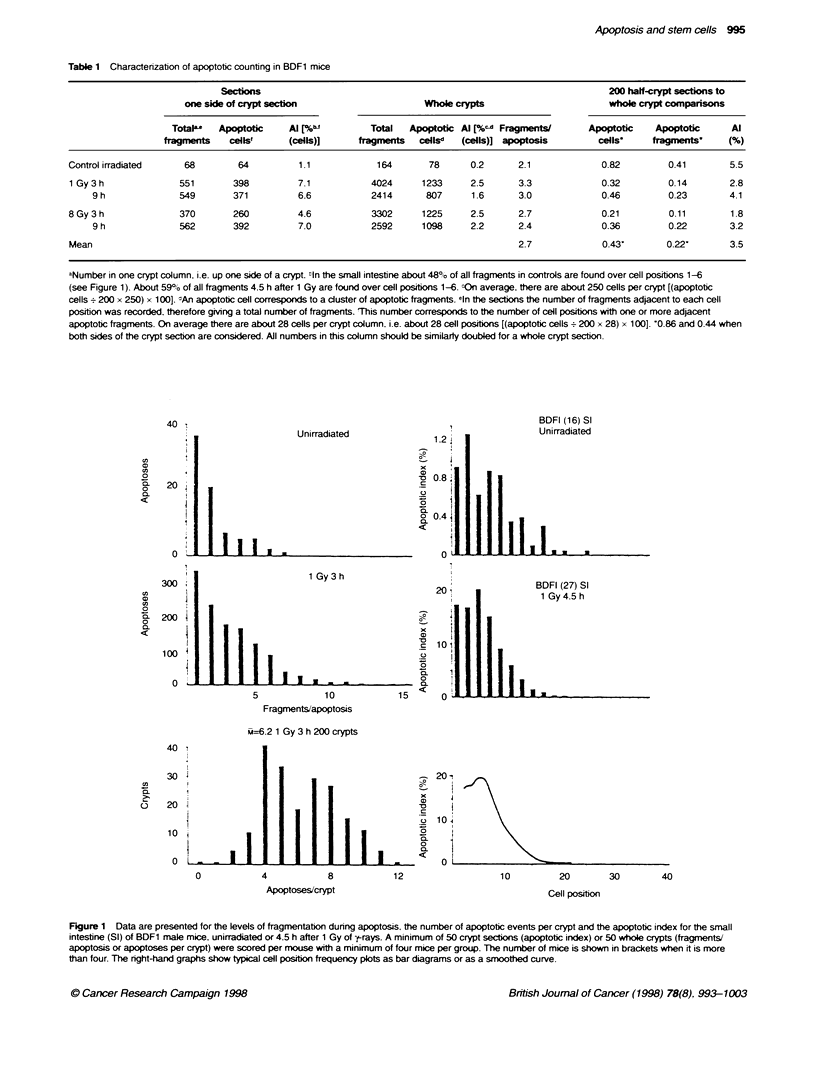
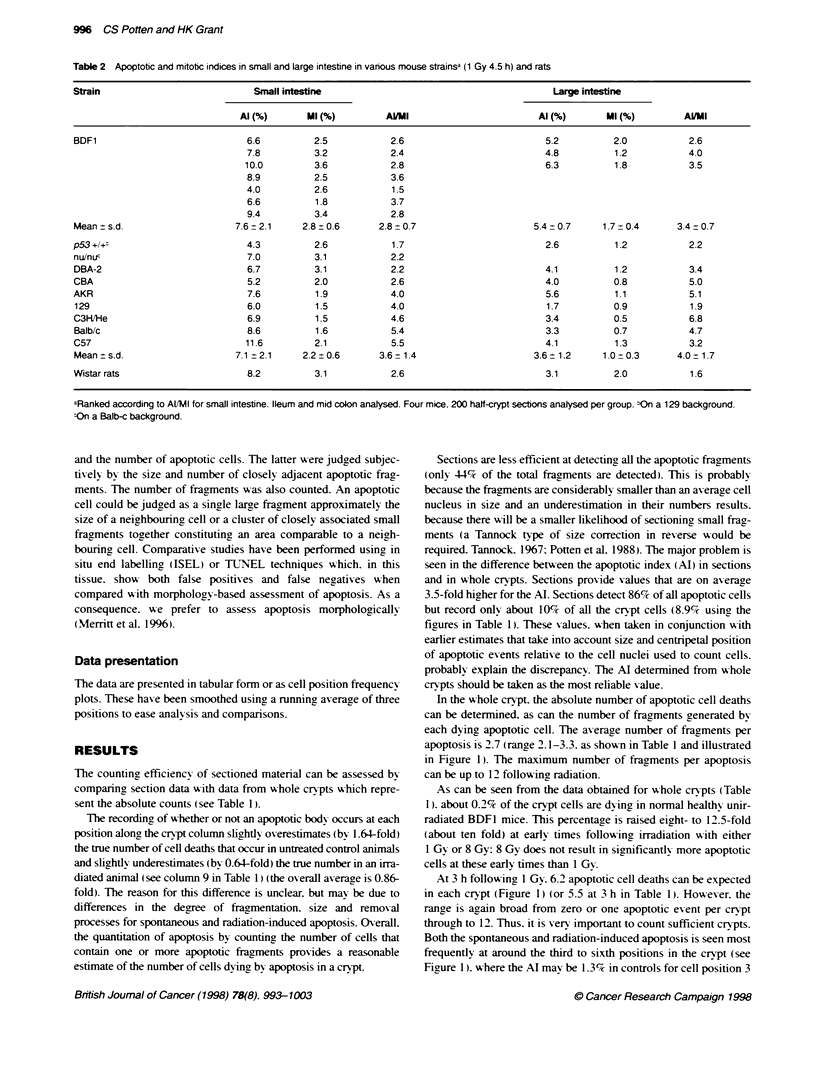
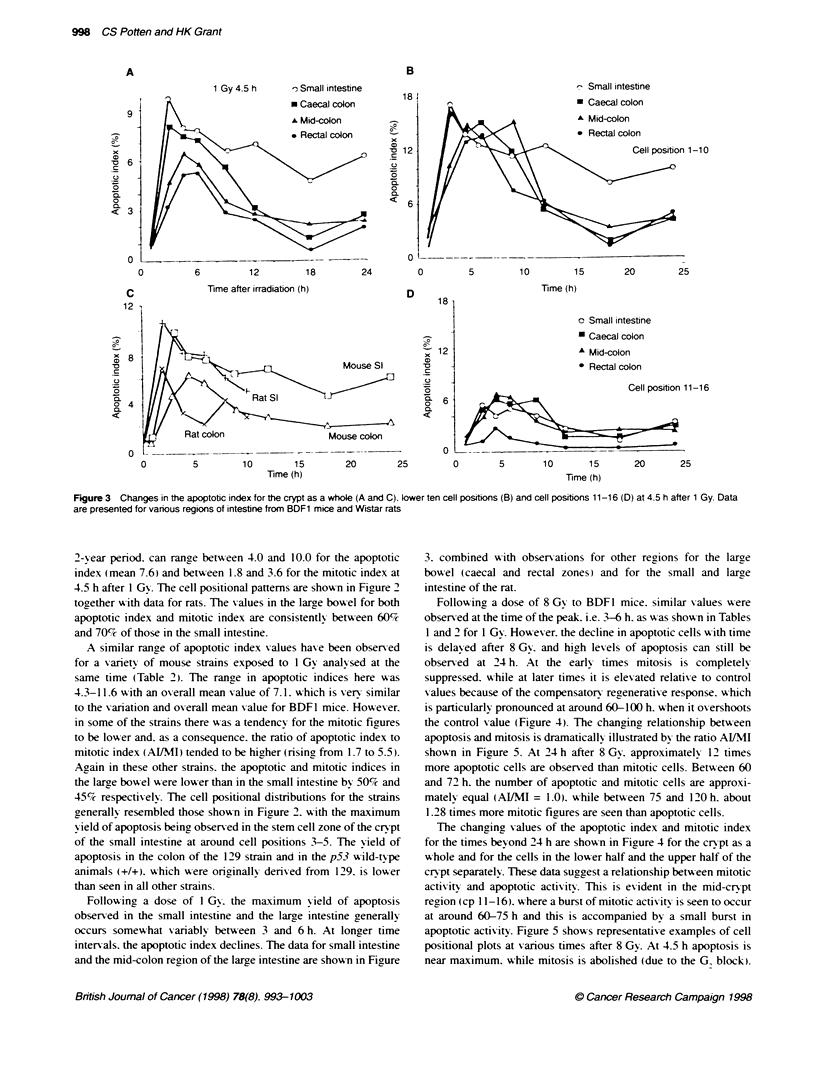
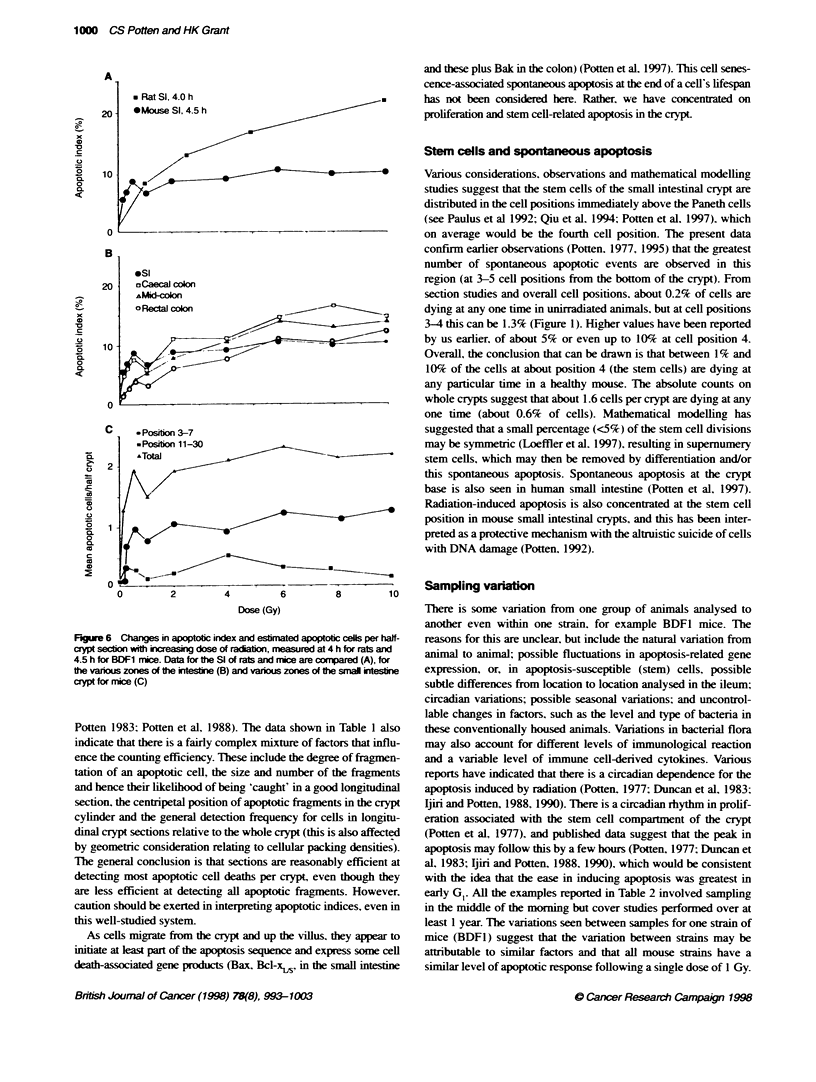
Apoptosis is observed in the crypts of the small intestine of healthy animals and man (spontaneous apoptosis). The levels can be dramatically elevated 3-6 h following ionizing radiation exposure. Both the spontaneous and radiation-induced apoptosis in the small intestine crypts are most frequently observed at the positions in the crypt associated with stem cells (about four cell positions from the base of the crypt). The number of apoptotic deaths can be counted in routine histological preparations, but interpretation of the counts is complicated by numerous factors. However, recording the number of cells containing one or more apoptotic fragments in crypt sections provides a good estimate for the absolute number of cell deaths in crypts. Similarities are noted in the frequency and cell positional relationship of radiation-induced apoptosis in the small intestine of various strains of mice and one strain of rat. Apoptosis in the large intestine is generally lower in frequency than in the small intestine and, for the mid-colonic and rectal regions, has a different cell positional frequency distribution, with the highest apoptotic yield at the crypt base. The caecal colon has a pattern of apoptotic distribution more similar to that in the small intestine. After exposure to 1 Gy ionizing radiation, the maximum apoptotic yield occurs over a period of 3-6 h in the small intestine. There is some unexplained variability in the values between groups of mice and between different mouse strains. After 8 Gy, the yield remains elevated for several days, however a similar maximum yield is still observed at the early times. In mouse large intestine and rat small intestine, the yield continues to rise until about 6 Gy in mouse large intestine and until at least 10 Gy in rat small intestine. Spontaneous apoptosis is interpreted as part of the homeostatic mechanism regulating stem cell numbers. About 1.6 cells per crypt are dying at any one time. Following irradiation, there is an apparent relationship between mitotic and apoptotic levels, suggesting that these processes are linked. The dose-response relationship suggests that there are about six apoptosis-susceptible cells in crypts of the small intestine, with about 2-4 of these occurring at cell positions in which there are other more resistant clonogenic cells. In the large intestine, the position of these apoptosis-susceptible cells varies with region, but the numbers are similar.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cai W. B., Roberts S. A., Potten C. S. The number of clonogenic cells in crypts in three regions of murine large intestine. Int J Radiat Biol. 1997 May;71(5):573–579. doi: 10.1080/095530097143905. [DOI] [PubMed] [Google Scholar]
- Duncan A. M., Ronen A., Blakey D. H. Diurnal variation in the response of gamma-ray-induced apoptosis in the mouse intestinal epithelium. Cancer Lett. 1983 Dec;21(2):163–166. doi: 10.1016/0304-3835(83)90203-3. [DOI] [PubMed] [Google Scholar]
- Hendry J. H., Potten C. S. Intestinal cell radiosensitivity: a comparison for cell death assayed by apoptosis or by a loss of clonogenicity. Int J Radiat Biol Relat Stud Phys Chem Med. 1982 Dec;42(6):621–628. doi: 10.1080/09553008214551601. [DOI] [PubMed] [Google Scholar]
- Ijiri K., Potten C. S. Circadian rhythms in the incidence of apoptotic cells and number of clonogenic cells in intestinal crypts after radiation using normal and reversed light conditions. Int J Radiat Biol Relat Stud Phys Chem Med. 1988 May;53(5):717–727. doi: 10.1080/09553008814551091. [DOI] [PubMed] [Google Scholar]
- Ijiri K., Potten C. S. Further studies on the response of intestinal crypt cells of different hierarchical status to eighteen different cytotoxic agents. Br J Cancer. 1987 Feb;55(2):113–123. doi: 10.1038/bjc.1987.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijiri K., Potten C. S. Response of intestinal cells of differing topographical and hierarchical status to ten cytotoxic drugs and five sources of radiation. Br J Cancer. 1983 Feb;47(2):175–185. doi: 10.1038/bjc.1983.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijiri K., Potten C. S. The circadian rhythm for the number and sensitivity of radiation-induced apoptosis in the crypts of mouse small intestine. Int J Radiat Biol. 1990 Jul;58(1):165–175. doi: 10.1080/09553009014551521. [DOI] [PubMed] [Google Scholar]
- Ijiri K., Potten C. S. The re-establishment of hypersensitive cells in the crypts of irradiated mouse intestine. Int J Radiat Biol Relat Stud Phys Chem Med. 1984 Nov;46(5):609–623. doi: 10.1080/09553008414551801. [DOI] [PubMed] [Google Scholar]
- Kovacs L., Potten C. S. An estimation of proliferative population size in stomach, jejunum and colon of DBA-2 mice. Cell Tissue Kinet. 1973 Mar;6(2):125–134. doi: 10.1111/j.1365-2184.1973.tb01601.x. [DOI] [PubMed] [Google Scholar]
- Li Y. Q., Fan C. Y., O'Connor P. J., Winton D. J., Potten C. S. Target cells for the cytotoxic effects of carcinogens in the murine small bowel. Carcinogenesis. 1992 Mar;13(3):361–368. doi: 10.1093/carcin/13.3.361. [DOI] [PubMed] [Google Scholar]
- Loeffler M., Bratke T., Paulus U., Li Y. Q., Potten C. S. Clonality and life cycles of intestinal crypts explained by a state dependent stochastic model of epithelial stem cell organization. J Theor Biol. 1997 May 7;186(1):41–54. doi: 10.1006/jtbi.1996.0340. [DOI] [PubMed] [Google Scholar]
- Merritt A. J., Allen T. D., Potten C. S., Hickman J. A. Apoptosis in small intestinal epithelial from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after gamma-irradiation. Oncogene. 1997 Jun 12;14(23):2759–2766. doi: 10.1038/sj.onc.1201126. [DOI] [PubMed] [Google Scholar]
- Merritt A. J., Potten C. S., Kemp C. J., Hickman J. A., Balmain A., Lane D. P., Hall P. A. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res. 1994 Feb 1;54(3):614–617. [PubMed] [Google Scholar]
- Merritt A. J., Potten C. S., Watson A. J., Loh D. Y., Nakayama K., Nakayama K., Hickman J. A. Differential expression of bcl-2 in intestinal epithelia. Correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci. 1995 Jun;108(Pt 6):2261–2271. doi: 10.1242/jcs.108.6.2261. [DOI] [PubMed] [Google Scholar]
- Paulus U., Potten C. S., Loeffler M. A model of the control of cellular regeneration in the intestinal crypt after perturbation based solely on local stem cell regulation. Cell Prolif. 1992 Nov;25(6):559–578. doi: 10.1111/j.1365-2184.1992.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Al-Barwari S. E., Hume W. J., Searle J. Circadian rhythms of presumptive stem cells in three different epithelia of the mouse. Cell Tissue Kinet. 1977 Nov;10(6):557–568. doi: 10.1111/j.1365-2184.1977.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Al-Barwari S. E., Searle J. Differential radiation response amongst proliferating epithelial cells. Cell Tissue Kinet. 1978 Mar;11(2):149–160. doi: 10.1111/j.1365-2184.1978.tb00883.x. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Booth C., Pritchard D. M. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol. 1997 Aug;78(4):219–243. doi: 10.1046/j.1365-2613.1997.280362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten C. S., Li Y. Q., O'Connor P. J., Winton D. J. A possible explanation for the differential cancer incidence in the intestine, based on distribution of the cytotoxic effects of carcinogens in the murine large bowel. Carcinogenesis. 1992 Dec;13(12):2305–2312. doi: 10.1093/carcin/13.12.2305. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990 Dec;110(4):1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Roberts S. A., Chwalinski S., Loeffler M., Paulus U. Scoring mitotic activity in longitudinal sections of crypts of the small intestine. Cell Tissue Kinet. 1988 Jul;21(4):231–246. doi: 10.1111/j.1365-2184.1988.tb00783.x. [DOI] [PubMed] [Google Scholar]
- Potten C. S. The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Cancer Metastasis Rev. 1992 Sep;11(2):179–195. doi: 10.1007/BF00048063. [DOI] [PubMed] [Google Scholar]
- Potten C. S. What is an apoptotic index measuring? A commentary. Br J Cancer. 1996 Dec;74(11):1743–1748. doi: 10.1038/bjc.1996.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard D. M., Watson A. J., Potten C. S., Jackman A. L., Hickman J. A. Inhibition by uridine but not thymidine of p53-dependent intestinal apoptosis initiated by 5-fluorouracil: evidence for the involvement of RNA perturbation. Proc Natl Acad Sci U S A. 1997 Mar 4;94(5):1795–1799. doi: 10.1073/pnas.94.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J. M., Roberts S. A., Potten C. S. Cell migration in the small and large bowel shows a strong circadian rhythm. Epithelial Cell Biol. 1994;3(4):137–148. [PubMed] [Google Scholar]
- Roberts S. A., Hendry J. H., Potten C. S. Deduction of the clonogen content of intestinal crypts: a direct comparison of two-dose and multiple-dose methodologies. Radiat Res. 1995 Mar;141(3):303–308. [PubMed] [Google Scholar]
- Sato M., Ahnen D. J. Regional variability of colonocyte growth and differentiation in the rat. Anat Rec. 1992 Jul;233(3):409–414. doi: 10.1002/ar.1092330308. [DOI] [PubMed] [Google Scholar]
- WIMBER D. E., QUASTLER H., STEIN O. L., WIMBER D. R. Analysis of tritium incorporation into individual cells by autoradiography of squash preparations. J Biophys Biochem Cytol. 1960 Oct;8:327–331. doi: 10.1083/jcb.8.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A. J., Merritt A. J., Jones L. S., Askew J. N., Anderson E., Becciolini A., Balzi M., Potten C. S., Hickman J. A. Evidence of reciprocity of bcl-2 and p53 expression in human colorectal adenomas and carcinomas. Br J Cancer. 1996 Apr;73(8):889–895. doi: 10.1038/bjc.1996.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil M. M., Stephens L. C., Amos C. I., Ruifrok A. C., Mason K. A. Strain difference in jejunal crypt cell susceptibility to radiation-induced apoptosis. Int J Radiat Biol. 1996 Nov;70(5):579–585. doi: 10.1080/095530096144789. [DOI] [PubMed] [Google Scholar]
- Withers H. R., Elkind M. M. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(3):261–267. doi: 10.1080/09553007014550291. [DOI] [PubMed] [Google Scholar]


