Abstract
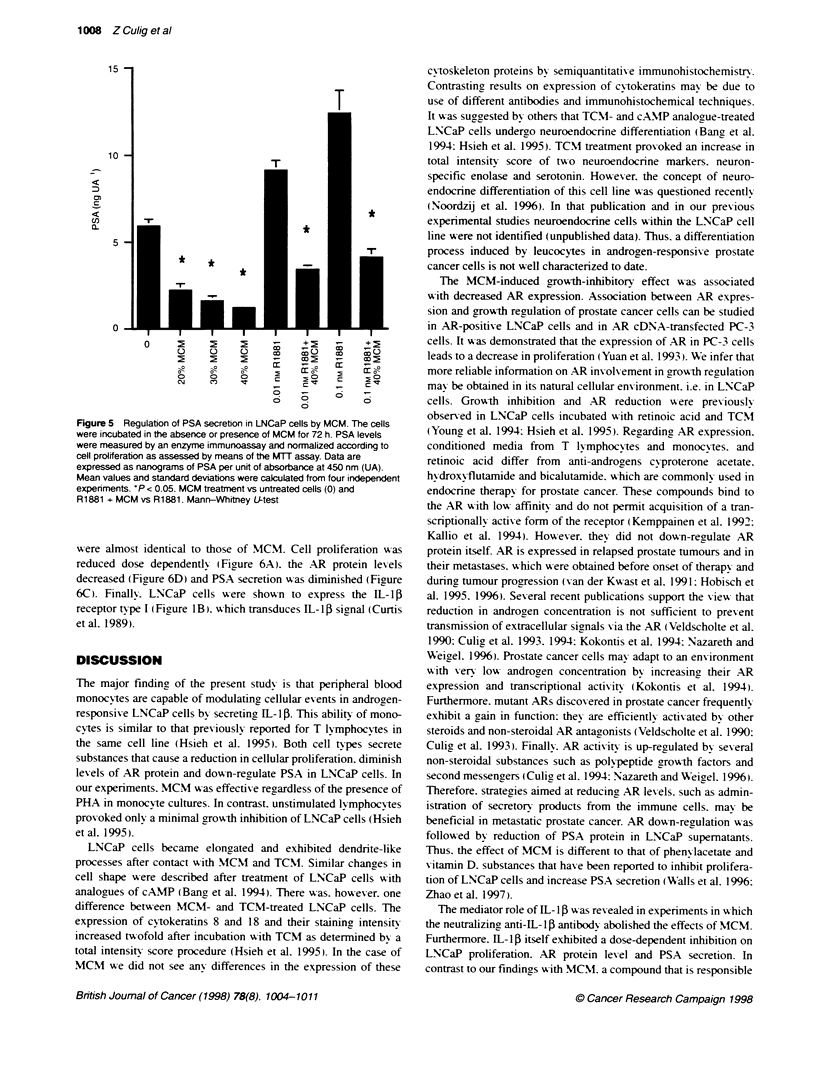
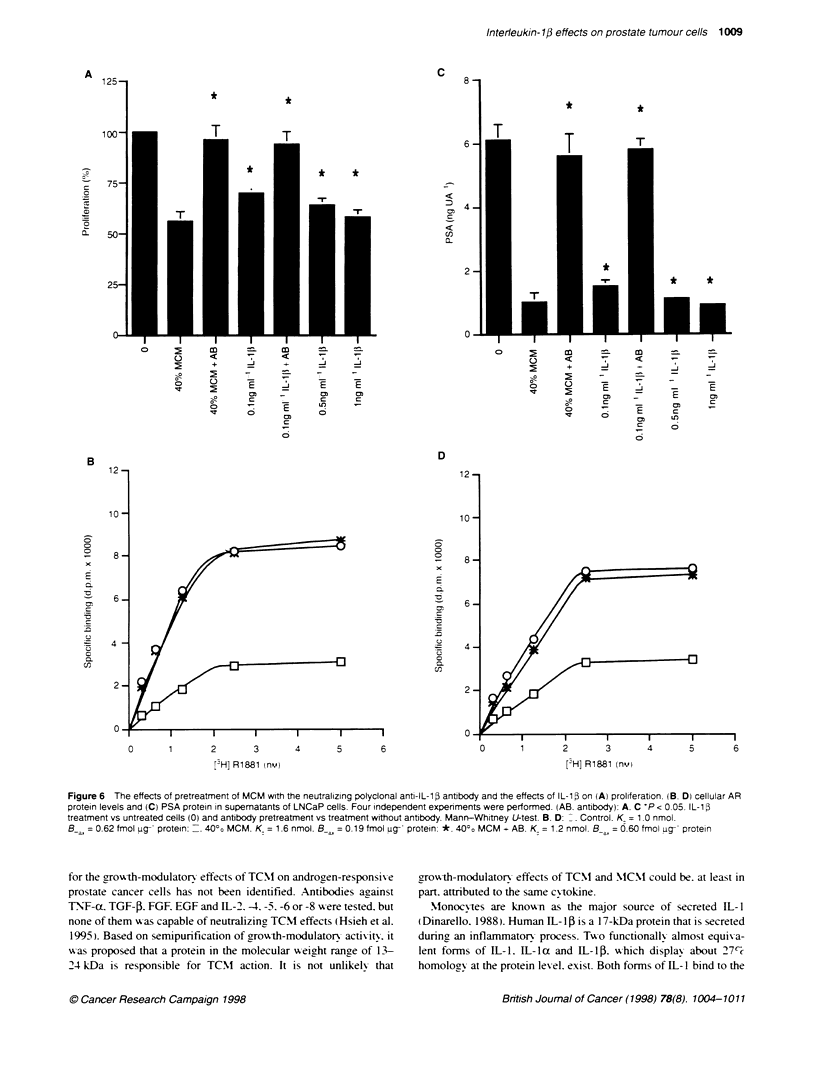
Proliferative and secretory responses in androgen-sensitive prostate cancer LNCaP cells are regulated by steroid and peptide hormones and by differentiation-promoting substances. In the present study, we evaluated whether peripheral blood monocytes that exhibit anti-tumour activity in haematopoietic and solid tumours influence growth and secretion in the LNCaP cell line. For this purpose, LNCaP cells were incubated with monocyte-conditioned medium (MCM), and proliferation as well as expression of androgen receptor (AR) and secretion of prostate-specific antigen (PSA) were assessed. Conditioned medium from monocytes reduced proliferation in a dose-dependent manner. Incubation with 40% MCM caused a 50% reduction in cell proliferation. AR protein decreased by 70% and PSA levels in supernatants from LNCaP cells were reduced by approximately 80% following treatment with MCM. We focused on the contribution of two major products of activated monocytes, prostaglandin E2 and interleukin 1beta (IL-1beta), to the MCM modulatory action. LNCaP cells treated with prostaglandin E2 showed neither a reduction in proliferation nor a down-regulation of AR and PSA levels. The effects of MCM on cellular proliferation, AR protein and PSA secretion were abolished by pretreatment of MCM with a neutralizing anti-IL-1beta antibody. In addition, recombinant IL-1beta was able to replace MCM for the inhibition of proliferation and down-regulation of AR and PSA proteins. LNCaP cells were shown to express the IL-1beta receptor type 1, which transduces IL-1beta signal. Our findings reveal that monocyte-derived IL-1beta inhibits the proliferation of androgen-responsive prostate tumour cells and reduces AR and PSA levels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bang Y. J., Pirnia F., Fang W. G., Kang W. K., Sartor O., Whitesell L., Ha M. J., Tsokos M., Sheahan M. D., Nguyen P. Terminal neuroendocrine differentiation of human prostate carcinoma cells in response to increased intracellular cyclic AMP. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5330–5334. doi: 10.1073/pnas.91.12.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braunschweiger P. G., Johnson C. S., Kumar N., Ord V., Furmanski P. Antitumor effects of recombinant human interleukin 1 alpha in RIF-1 and Panc02 solid tumors. Cancer Res. 1988 Nov 1;48(21):6011–6016. [PubMed] [Google Scholar]
- Carman-Krzan M., Wise B. C. Arachidonic acid lipoxygenation may mediate interleukin-1 stimulation of nerve growth factor secretion in astroglial cultures. J Neurosci Res. 1993 Feb 1;34(2):225–232. doi: 10.1002/jnr.490340210. [DOI] [PubMed] [Google Scholar]
- Cole O. F., Seki H., Elder M. G., Sullivan M. H. Interleukin-1 beta independently stimulates production of prostaglandin E2 and cyclic AMP from human decidual cells. Biochim Biophys Acta. 1995 Nov 9;1269(2):139–144. doi: 10.1016/0167-4889(95)00107-4. [DOI] [PubMed] [Google Scholar]
- Cronauer M. V., Klocker H., Talasz H., Geisen F. H., Hobisch A., Radmayr C., Böck G., Culig Z., Schirmer M., Reissigl A. Inhibitory effects of the nucleoside analogue gemcitabine on prostatic carcinoma cells. Prostate. 1996 Mar;28(3):172–181. doi: 10.1002/(SICI)1097-0045(199603)28:3<172::AID-PROS4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Culig Z., Hobisch A., Cronauer M. V., Cato A. C., Hittmair A., Radmayr C., Eberle J., Bartsch G., Klocker H. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol Endocrinol. 1993 Dec;7(12):1541–1550. doi: 10.1210/mend.7.12.8145761. [DOI] [PubMed] [Google Scholar]
- Culig Z., Hobisch A., Cronauer M. V., Radmayr C., Trapman J., Hittmair A., Bartsch G., Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994 Oct 15;54(20):5474–5478. [PubMed] [Google Scholar]
- Curtis B. M., Gallis B., Overell R. W., McMahan C. J., DeRoos P., Ireland R., Eisenman J., Dower S. K., Sims J. E. T-cell interleukin 1 receptor cDNA expressed in Chinese hamster ovary cells regulates functional responses to interleukin 1. Proc Natl Acad Sci U S A. 1989 May;86(9):3045–3049. doi: 10.1073/pnas.86.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danforth D. N., Jr, Sgagias M. K. Interleukin 1 alpha blocks estradiol-stimulated growth and down-regulates the estrogen receptor in MCF-7 breast cancer cells in vitro. Cancer Res. 1991 Mar 1;51(5):1488–1493. [PubMed] [Google Scholar]
- Esquenet M., Swinnen J. V., Heyns W., Verhoeven G. Triiodothyronine modulates growth, secretory function and androgen receptor concentration in the prostatic carcinoma cell line LNCaP. Mol Cell Endocrinol. 1995 Mar;109(1):105–111. doi: 10.1016/0303-7207(95)03490-x. [DOI] [PubMed] [Google Scholar]
- Fong C. J., Sutkowski D. M., Braun E. J., Bauer K. D., Sherwood E. R., Lee C., Kozlowski J. M. Effect of retinoic acid on the proliferation and secretory activity of androgen-responsive prostatic carcinoma cells. J Urol. 1993 May;149(5):1190–1194. doi: 10.1016/s0022-5347(17)36345-0. [DOI] [PubMed] [Google Scholar]
- Hobisch A., Culig Z., Radmayr C., Bartsch G., Klocker H., Hittmair A. Distant metastases from prostatic carcinoma express androgen receptor protein. Cancer Res. 1995 Jul 15;55(14):3068–3072. [PubMed] [Google Scholar]
- Horoszewicz J. S., Leong S. S., Kawinski E., Karr J. P., Rosenthal H., Chu T. M., Mirand E. A., Murphy G. P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983 Apr;43(4):1809–1818. [PubMed] [Google Scholar]
- Hsieh T. C., Xu W., Chiao J. W. Growth regulation and cellular changes during differentiation of human prostatic cancer LNCaP cells as induced by T lymphocyte-conditioned medium. Exp Cell Res. 1995 May;218(1):137–143. doi: 10.1006/excr.1995.1140. [DOI] [PubMed] [Google Scholar]
- Iversen P. O., Hart P. H., Bonder C. S., Lopez A. F. Interleukin (IL)-10, but not IL-4 or IL-13, inhibits cytokine production and growth in juvenile myelomonocytic leukemia cells. Cancer Res. 1997 Feb 1;57(3):476–480. [PubMed] [Google Scholar]
- Kallio P. J., Jänne O. A., Palvimo J. J. Agonists, but not antagonists, alter the conformation of the hormone-binding domain of androgen receptor. Endocrinology. 1994 Feb;134(2):998–1001. doi: 10.1210/endo.134.2.8299593. [DOI] [PubMed] [Google Scholar]
- Kemppainen J. A., Lane M. V., Sar M., Wilson E. M. Androgen receptor phosphorylation, turnover, nuclear transport, and transcriptional activation. Specificity for steroids and antihormones. J Biol Chem. 1992 Jan 15;267(2):968–974. [PubMed] [Google Scholar]
- Kilian P. L., Kaffka K. L., Biondi D. A., Lipman J. M., Benjamin W. R., Feldman D., Campen C. A. Antiproliferative effect of interleukin-1 on human ovarian carcinoma cell line (NIH:OVCAR-3). Cancer Res. 1991 Apr 1;51(7):1823–1828. [PubMed] [Google Scholar]
- Kim I. Y., Kim J. H., Zelner D. J., Ahn H. J., Sensibar J. A., Lee C. Transforming growth factor-beta1 is a mediator of androgen-regulated growth arrest in an androgen-responsive prostatic cancer cell line, LNCaP. Endocrinology. 1996 Mar;137(3):991–999. doi: 10.1210/endo.137.3.8603613. [DOI] [PubMed] [Google Scholar]
- Klein R. D., Borchers A. H., Sundareshan P., Bougelet C., Berkman M. R., Nagle R. B., Bowden G. T. Interleukin-1beta secreted from monocytic cells induces the expression of matrilysin in the prostatic cell line LNCaP. J Biol Chem. 1997 May 30;272(22):14188–14192. doi: 10.1074/jbc.272.22.14188. [DOI] [PubMed] [Google Scholar]
- Kokontis J., Takakura K., Hay N., Liao S. Increased androgen receptor activity and altered c-myc expression in prostate cancer cells after long-term androgen deprivation. Cancer Res. 1994 Mar 15;54(6):1566–1573. [PubMed] [Google Scholar]
- Lee C., Sutkowski D. M., Sensibar J. A., Zelner D., Kim I., Amsel I., Shaw N., Prins G. S., Kozlowski J. M. Regulation of proliferation and production of prostate-specific antigen in androgen-sensitive prostatic cancer cells, LNCaP, by dihydrotestosterone. Endocrinology. 1995 Feb;136(2):796–803. doi: 10.1210/endo.136.2.7530653. [DOI] [PubMed] [Google Scholar]
- Limonta P., Dondi D., Moretti R. M., Maggi R., Motta M. Antiproliferative effects of luteinizing hormone-releasing hormone agonists on the human prostatic cancer cell line LNCaP. J Clin Endocrinol Metab. 1992 Jul;75(1):207–212. doi: 10.1210/jcem.75.1.1320049. [DOI] [PubMed] [Google Scholar]
- Lisak R. P., Bealmear B. Antibodies to interleukin-1 inhibit cytokine-induced proliferation of neonatal rat Schwann cells in vitro. J Neuroimmunol. 1991 Feb;31(2):123–132. doi: 10.1016/0165-5728(91)90018-3. [DOI] [PubMed] [Google Scholar]
- MacDonald A., Habib F. K. Divergent responses to epidermal growth factor in hormone sensitive and insensitive human prostate cancer cell lines. Br J Cancer. 1992 Feb;65(2):177–182. doi: 10.1038/bjc.1992.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz E., Beutner U., Zubiaga A., Huber B. T. IL-1 activates two separate signal transduction pathways in T helper type II cells. J Immunol. 1990 Feb 1;144(3):964–969. [PubMed] [Google Scholar]
- Nakamoto T., Chang C. S., Li A. K., Chodak G. W. Basic fibroblast growth factor in human prostate cancer cells. Cancer Res. 1992 Feb 1;52(3):571–577. [PubMed] [Google Scholar]
- Nazareth L. V., Weigel N. L. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem. 1996 Aug 16;271(33):19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- Noordzij M. A., van Weerden W. M., de Ridder C. M., van der Kwast T. H., Schröder F. H., van Steenbrugge G. J. Neuroendocrine differentiation in human prostatic tumor models. Am J Pathol. 1996 Sep;149(3):859–871. [PMC free article] [PubMed] [Google Scholar]
- Peehl D. M., Wong S. T., Stamey T. A. Vitamin A regulates proliferation and differentiation of human prostatic epithelial cells. Prostate. 1993;23(1):69–78. doi: 10.1002/pros.2990230107. [DOI] [PubMed] [Google Scholar]
- Powell W. C., Knox J. D., Navre M., Grogan T. M., Kittelson J., Nagle R. B., Bowden G. T. Expression of the metalloproteinase matrilysin in DU-145 cells increases their invasive potential in severe combined immunodeficient mice. Cancer Res. 1993 Jan 15;53(2):417–422. [PubMed] [Google Scholar]
- Ritchie C. K., Andrews L. R., Thomas K. G., Tindall D. J., Fitzpatrick L. A. The effects of growth factors associated with osteoblasts on prostate carcinoma proliferation and chemotaxis: implications for the development of metastatic disease. Endocrinology. 1997 Mar;138(3):1145–1150. doi: 10.1210/endo.138.3.4974. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Vodovotz Y., Roche N. S., Sporn M. B., Nathan C. F. Role of nitric oxide in antagonistic effects of transforming growth factor-beta and interleukin-1 beta on the beating rate of cultured cardiac myocytes. Mol Endocrinol. 1992 Nov;6(11):1921–1930. doi: 10.1210/mend.6.11.1282674. [DOI] [PubMed] [Google Scholar]
- Salem P., Deryckx S., Dulioust A., Vivier E., Denizot Y., Damais C., Dinarello C. A., Thomas Y. Immunoregulatory functions of paf-acether. IV. Enhancement of IL-1 production by muramyl dipeptide-stimulated monocytes. J Immunol. 1990 Feb 15;144(4):1338–1344. [PubMed] [Google Scholar]
- Skowronski R. J., Peehl D. M., Feldman D. Vitamin D and prostate cancer: 1,25 dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology. 1993 May;132(5):1952–1960. doi: 10.1210/endo.132.5.7682937. [DOI] [PubMed] [Google Scholar]
- Veldscholte J., Ris-Stalpers C., Kuiper G. G., Jenster G., Berrevoets C., Claassen E., van Rooij H. C., Trapman J., Brinkmann A. O., Mulder E. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990 Dec 14;173(2):534–540. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- Venkataprasad N., Shiratsuchi H., Johnson J. L., Ellner J. J. Induction of prostaglandin E2 by human monocytes infected with Mycobacterium avium complex--modulation of cytokine expression. J Infect Dis. 1996 Oct;174(4):806–811. doi: 10.1093/infdis/174.4.806. [DOI] [PubMed] [Google Scholar]
- Walls R., Thibault A., Liu L., Wood C., Kozlowski J. M., Figg W. D., Sampson M. L., Elin R. J., Samid D. The differentiating agent phenylacetate increases prostate-specific antigen production by prostate cancer cells. Prostate. 1996 Sep;29(3):177–182. doi: 10.1002/(SICI)1097-0045(199609)29:3<177::AID-PROS3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Wang J., Huang M., Lee P., Komanduri K., Sharma S., Chen G., Dubinett S. M. Interleukin-8 inhibits non-small cell lung cancer proliferation: a possible role for regulation of tumor growth by autocrine and paracrine pathways. J Interferon Cytokine Res. 1996 Jan;16(1):53–60. doi: 10.1089/jir.1996.16.53. [DOI] [PubMed] [Google Scholar]
- Wilding G., Valverius E., Knabbe C., Gelmann E. P. Role of transforming growth factor-alpha in human prostate cancer cell growth. Prostate. 1989;15(1):1–12. doi: 10.1002/pros.2990150102. [DOI] [PubMed] [Google Scholar]
- Young C. Y., Murtha P. E., Andrews P. E., Lindzey J. K., Tindall D. J. Antagonism of androgen action in prostate tumor cells by retinoic acid. Prostate. 1994 Jul;25(1):39–45. doi: 10.1002/pros.2990250106. [DOI] [PubMed] [Google Scholar]
- Yuan S., Trachtenberg J., Mills G. B., Brown T. J., Xu F., Keating A. Androgen-induced inhibition of cell proliferation in an androgen-insensitive prostate cancer cell line (PC-3) transfected with a human androgen receptor complementary DNA. Cancer Res. 1993 Mar 15;53(6):1304–1311. [PubMed] [Google Scholar]
- Zhao X. Y., Ly L. H., Peehl D. M., Feldman D. 1alpha,25-dihydroxyvitamin D3 actions in LNCaP human prostate cancer cells are androgen-dependent. Endocrinology. 1997 Aug;138(8):3290–3298. doi: 10.1210/endo.138.8.5328. [DOI] [PubMed] [Google Scholar]
- van der Kwast T. H., Schalken J., Ruizeveld de Winter J. A., van Vroonhoven C. C., Mulder E., Boersma W., Trapman J. Androgen receptors in endocrine-therapy-resistant human prostate cancer. Int J Cancer. 1991 May 10;48(2):189–193. doi: 10.1002/ijc.2910480206. [DOI] [PubMed] [Google Scholar]