Abstract
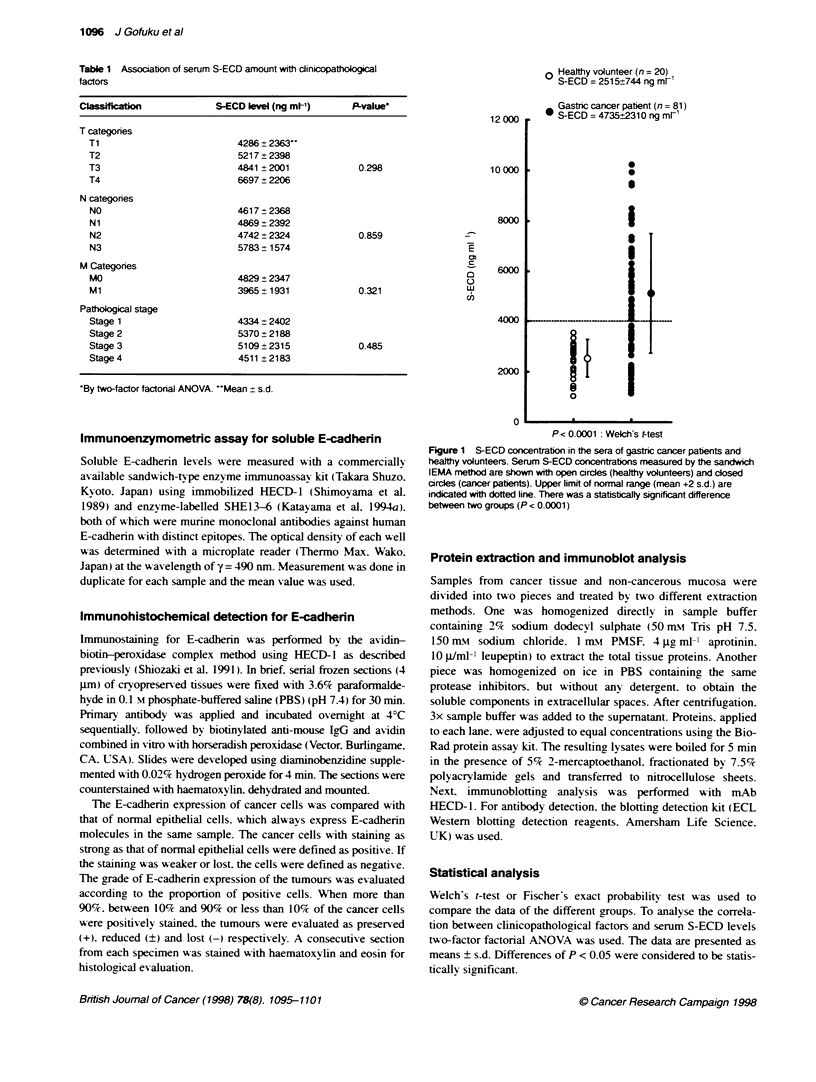
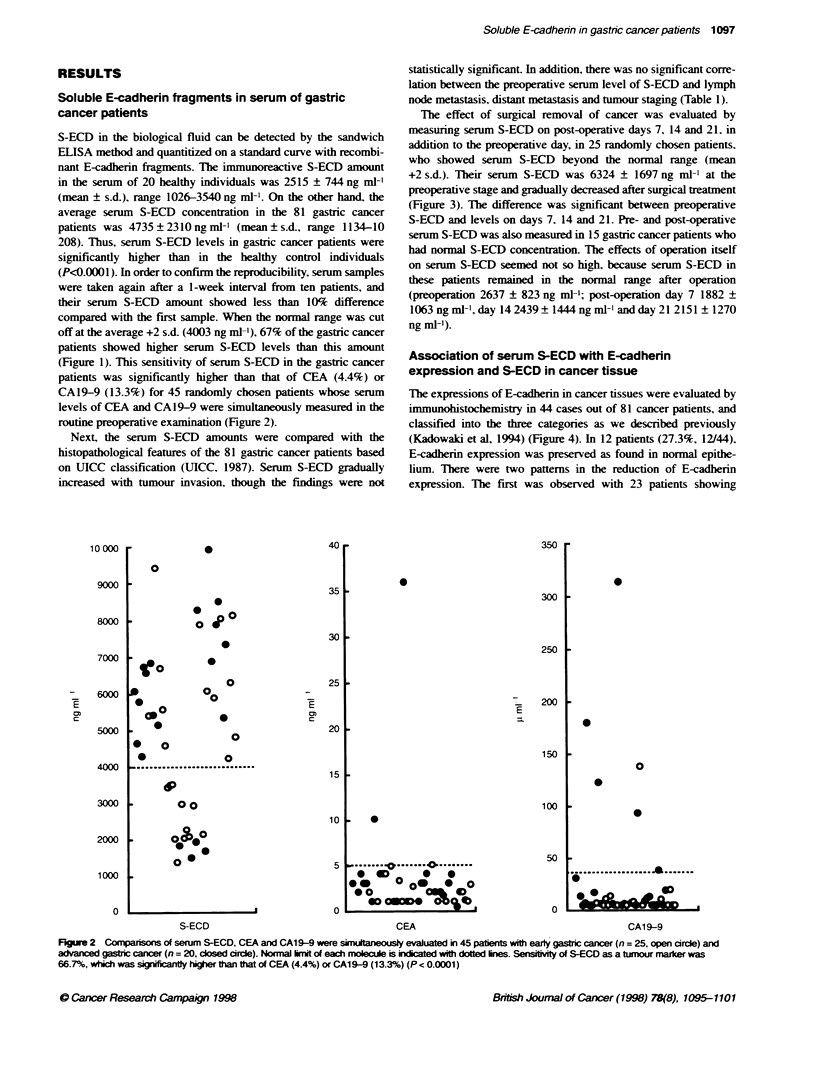
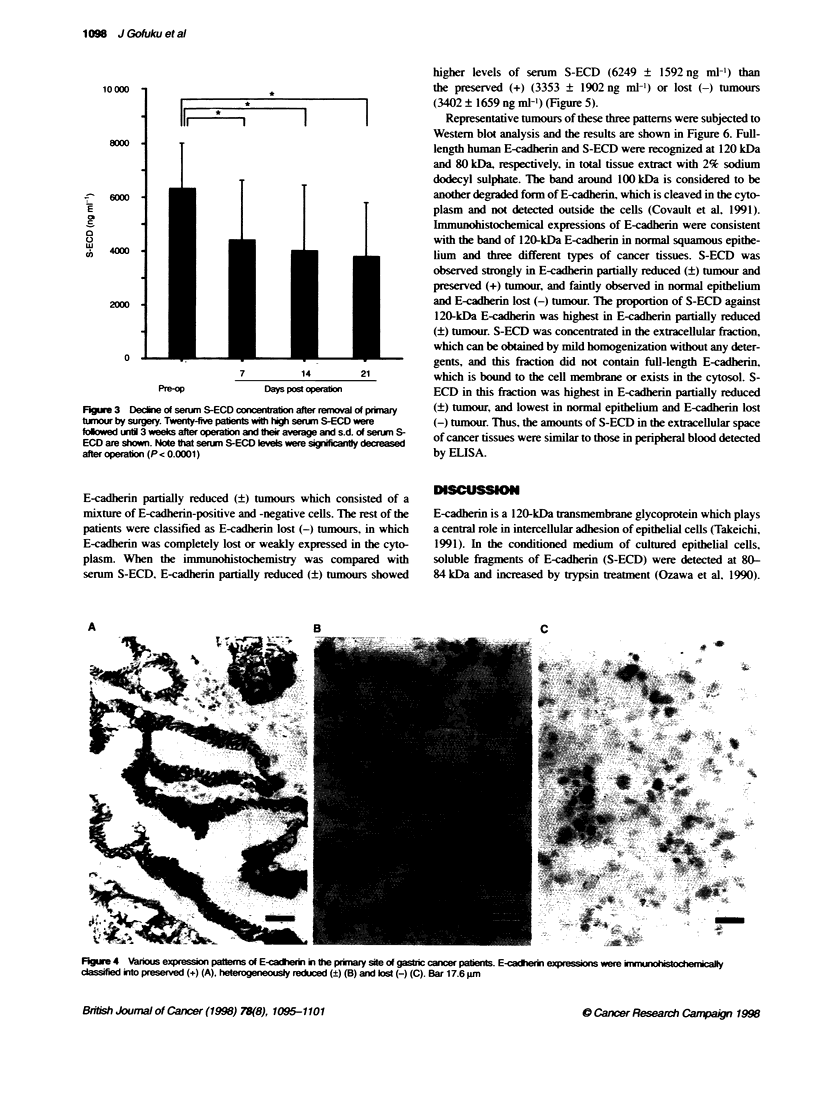
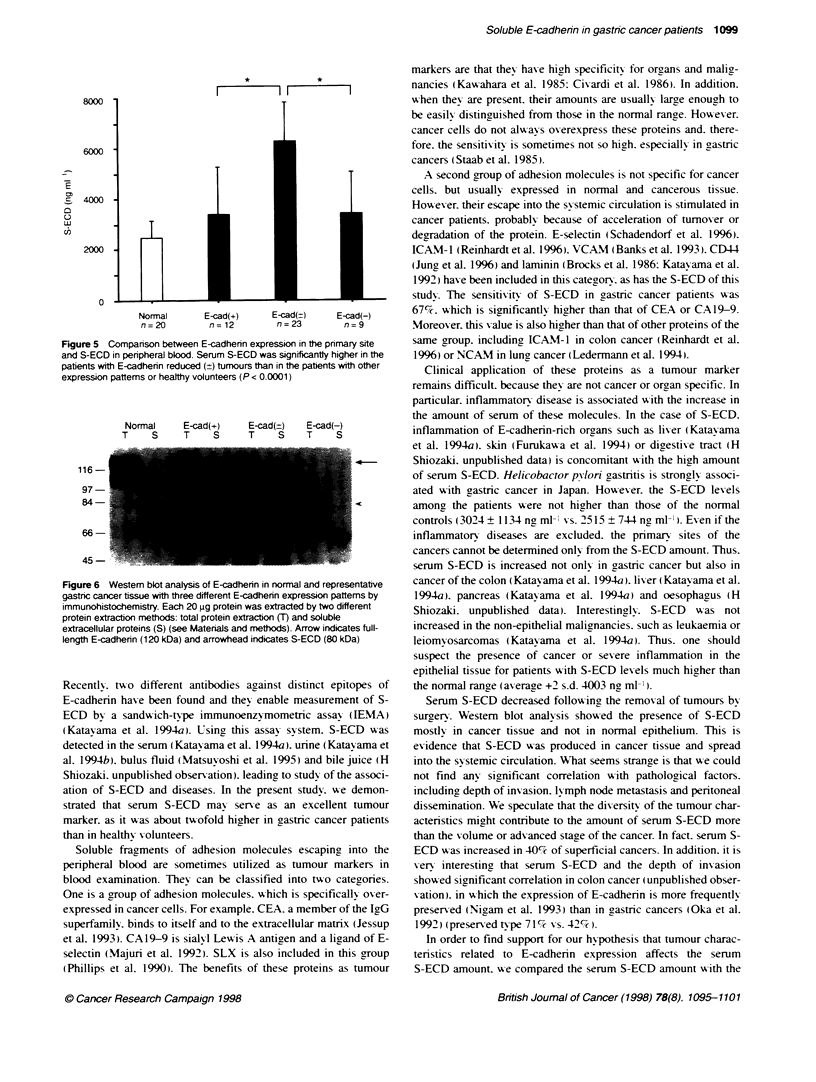
The soluble fragment of E-cadherin protein (S-ECD) is reported to be increased in the peripheral blood of cancer patients. In this study, we investigated the clinical significance of serum S-ECD in 81 patients with gastric cancer. The amount of serum S-ECD was significantly higher in the gastric cancer patients (4735 +/- 2310 ng ml(-1)) than in healthy volunteers (2515 +/- 744 ng ml(-1)). With the normal range cut-off at average +2 s.d., 67% patients showed abnormally high serum S-ECD levels. This frequency was significantly higher than that of other tumour markers, such as CEA (4.4%) or CA19-9 (13.3%). However, there was no significant correlation between the amount of S-ECD and clinicopathological factors. Serum S-ECD might be derived from cancer tissue, as removal of cancers by surgical treatment results in quick decline of the serum S-ECD and S-ECD can be detected by immunoblot in cancer tissues but not in normal epithelium. The serum S-ECD amount was compared with the E-cadherin expression in cancer tissues, which were classified into those showing preserved (+), partially reduced (+/-) or lost (-) expression. Interestingly, E-cadherin (+/-) tumours showed higher serum S-ECD levels than the other types, and a higher amount of S-ECD in the immunoblot analysis. Thus, the serum level of S-ECD may serve as an excellent tumour marker with high sensitivity. Furthermore, analysis of S-ECD in serum and cancer tissue can offer clues for elucidating the mechanism of reduction of E-cadherin expression in cancer cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Nigam A. K., Savage F. J., Boulos P. B., Stamp G. W., Liu D., Pignatelli M. Loss of cell-cell and cell-matrix adhesion molecules in colorectal cancer. Br J Cancer. 1993 Sep;68(3):507–514. doi: 10.1038/bjc.1993.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka H., Shiozaki H., Kobayashi K., Tahara H., Tamura S., Miyata M., Doki Y., Iihara K., Matsuyoshi N., Hirano S. Immunohistochemical evaluation of E-cadherin adhesion molecule expression in human gastric cancer. Virchows Arch A Pathol Anat Histopathol. 1992;421(2):149–156. doi: 10.1007/BF01607048. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Baribault H., Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989 Jun;8(6):1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Engel J., Kemler R. Single amino acid substitutions in one Ca2+ binding site of uvomorulin abolish the adhesive function. Cell. 1990 Nov 30;63(5):1033–1038. doi: 10.1016/0092-8674(90)90506-a. [DOI] [PubMed] [Google Scholar]
- Peyriéras N., Hyafil F., Louvard D., Ploegh H. L., Jacob F. Uvomorulin: a nonintegral membrane protein of early mouse embryo. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6274–6277. doi: 10.1073/pnas.80.20.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990 Nov 23;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Reinhardt K. M., Steiner M., Zillig D., Nagel H. R., Blann A. D., Brinckmann W. Soluble intercellular adhesion molecule-1 in colorectal cancer and its relationship to acute phase proteins. Neoplasma. 1996;43(2):65–67. [PubMed] [Google Scholar]
- Schadendorf D., Diehl S., Zuberbier T., Schadendorf C., Henz B. M. Quantitative detection of soluble adhesion molecules in sera of melanoma patients correlates with clinical stage. Dermatology. 1996;192(2):89–93. doi: 10.1159/000246328. [DOI] [PubMed] [Google Scholar]
- Shiozaki H., Oka H., Inoue M., Tamura S., Monden M. E-cadherin mediated adhesion system in cancer cells. Cancer. 1996 Apr 15;77(8 Suppl):1605–1613. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1605::AID-CNCR28>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Shiozaki H., Tahara H., Oka H., Miyata M., Kobayashi K., Tamura S., Iihara K., Doki Y., Hirano S., Takeichi M. Expression of immunoreactive E-cadherin adhesion molecules in human cancers. Am J Pathol. 1991 Jul;139(1):17–23. [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991 Mar 22;251(5000):1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Vessey C. J., Wilding J., Folarin N., Hirano S., Takeichi M., Soutter P., Stamp G. W., Pignatelli M. Altered expression and function of E-cadherin in cervical intraepithelial neoplasia and invasive squamous cell carcinoma. J Pathol. 1995 Jun;176(2):151–159. doi: 10.1002/path.1711760208. [DOI] [PubMed] [Google Scholar]
- Yoshiura K., Kanai Y., Ochiai A., Shimoyama Y., Sugimura T., Hirohashi S. Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7416–7419. doi: 10.1073/pnas.92.16.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]