Abstract
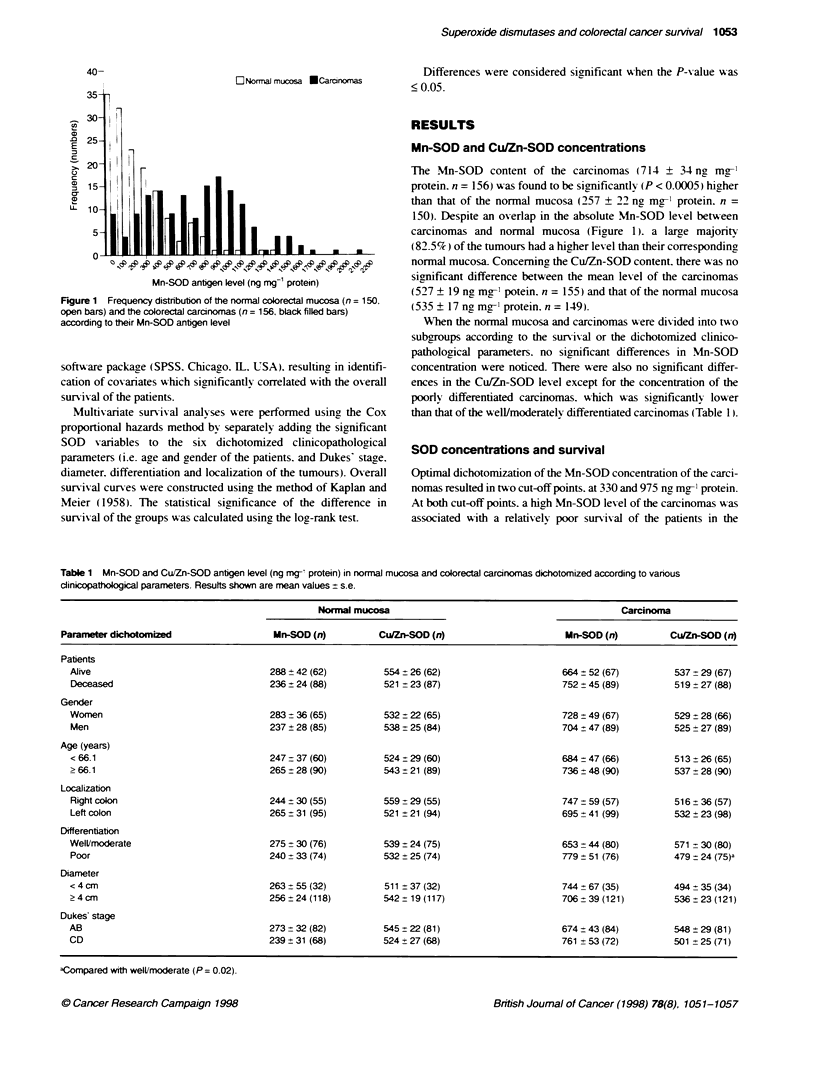
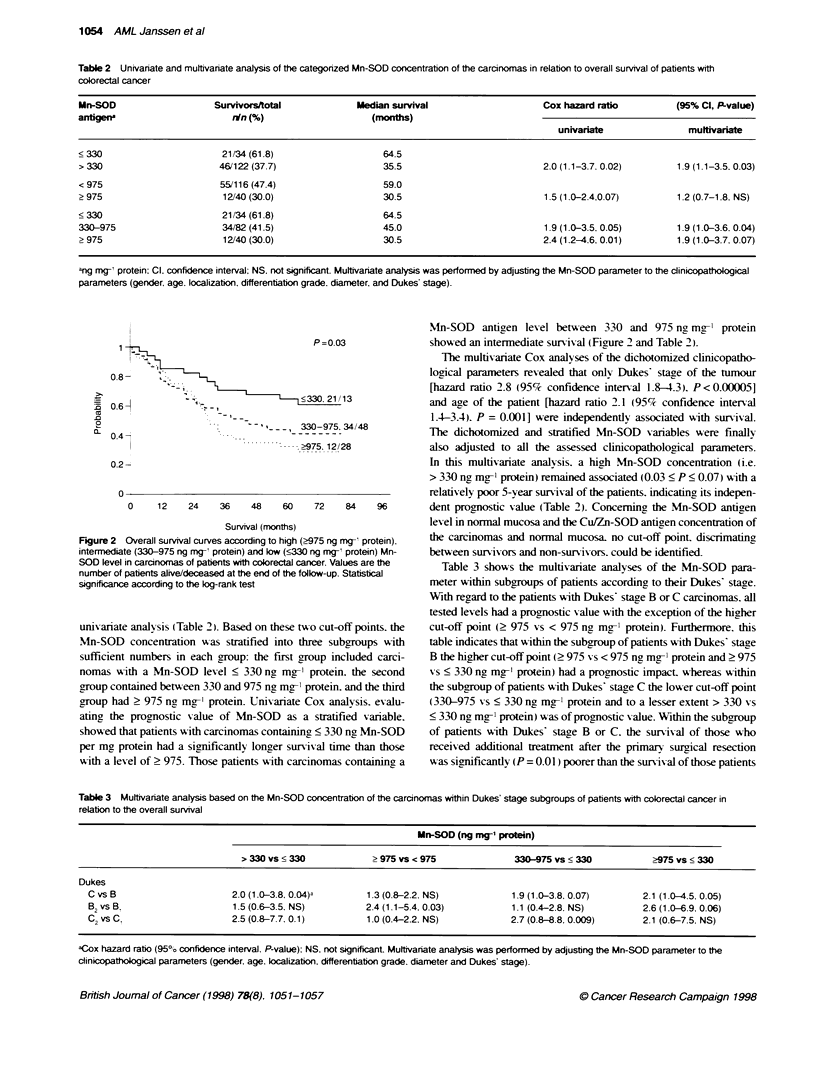
Reactive oxygen metabolites are implicated in the initiation and promotion of cancer. In addition, oxidant scavengers, such as manganese--(Mn-SOD) and copper/zinc--superoxide dismutase (Cu/Zn-SOD), are thought to contribute to colorectal cancer treatment response. In the present study, the prognostic significance of the Mn- and Cu/Zn-SOD antigen content of normal mucosa and carcinomas of 163 patients with colorectal cancer was evaluated in comparison with major clinicopathological parameters, with respect to the 5-year overall survival. The Mn-SOD content of carcinomas was found to be significantly higher than that of normal mucosa, whereas there was no difference in the Cu/Zn-SOD content between the normal mucosa and carcinomas. No association was demonstrable between the Mn-SOD and Cu/Zn-SOD content of the tissues and the assessed clinicopathological parameters (gender, age, localization, differentiation grade, diameter and Dukes' stage), with the exception of the Cu/Zn-SOD and the differentiation grade of the carcinomas. Univariate analysis showed that a high Mn-SOD content of carcinomas was associated with a poor 5-year overall survival of the patients with colorectal cancer. Multivariate analysis including all clinicopathological parameters revealed that this Mn-SOD parameter was prognostically independent. The Mn- and Cu/Zn-SOD content of normal mucosa and the Cu/Zn-SOD content of carcinomas were not associated with the overall survival of the patients. In conclusion, this study demonstrates that for patients with colorectal cancer the Mn-SOD content of colorectal carcinomas has a significant prognostic value that is independent from major clinicopathological parameters, including Dukes' stage.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTLER V. B., COLLER F. A. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg. 1954 Jun;139(6):846–852. doi: 10.1097/00000658-195406000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beahrs O. H. Staging of cancer of the colon and rectum. Cancer. 1992 Sep 1;70(5 Suppl):1393–1396. doi: 10.1002/1097-0142(19920901)70:3+<1393::aid-cncr2820701530>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Beyer W., Imlay J., Fridovich I. Superoxide dismutases. Prog Nucleic Acid Res Mol Biol. 1991;40:221–253. doi: 10.1016/s0079-6603(08)60843-0. [DOI] [PubMed] [Google Scholar]
- Borek C. Radiation and chemically induced transformation: free radicals, antioxidants and cancer. Br J Cancer Suppl. 1987 Jun;8:74–86. [PMC free article] [PubMed] [Google Scholar]
- Deans G. T., Parks T. G., Rowlands B. J., Spence R. A. Prognostic factors in colorectal cancer. Br J Surg. 1992 Jul;79(7):608–613. doi: 10.1002/bjs.1800790706. [DOI] [PubMed] [Google Scholar]
- Eastgate J., Moreb J., Nick H. S., Suzuki K., Taniguchi N., Zucali J. R. A role for manganese superoxide dismutase in radioprotection of hematopoietic stem cells by interleukin-1. Blood. 1993 Feb 1;81(3):639–646. [PubMed] [Google Scholar]
- Farber J. L., Kyle M. E., Coleman J. B. Mechanisms of cell injury by activated oxygen species. Lab Invest. 1990 Jun;62(6):670–679. [PubMed] [Google Scholar]
- Farber J. L. Mechanisms of cell injury by activated oxygen species. Environ Health Perspect. 1994 Dec;102 (Suppl 10):17–24. doi: 10.1289/ehp.94102s1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Glover J. Free radical biology: a paradox in cancer research. J Natl Cancer Inst. 1990 Jun 6;82(11):902–903. doi: 10.1093/jnci/82.11.902. [DOI] [PubMed] [Google Scholar]
- Greenwald P. Colon cancer overview. Cancer. 1992 Sep 1;70(5 Suppl):1206–1215. doi: 10.1002/1097-0142(19920901)70:3+<1206::aid-cncr2820701504>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Guyton K. Z., Kensler T. W. Oxidative mechanisms in carcinogenesis. Br Med Bull. 1993 Jul;49(3):523–544. doi: 10.1093/oxfordjournals.bmb.a072628. [DOI] [PubMed] [Google Scholar]
- Götz J. M., van Kan C. I., Verspaget H. W., Biemond I., Lamers C. B., Veenendaal R. A. Gastric mucosal superoxide dismutases in Helicobacter pylori infection. Gut. 1996 Apr;38(4):502–506. doi: 10.1136/gut.38.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser G. J., McIntosh J. K., Travis W. D., Rosenberg S. A. Manipulation of oxygen radical-scavenging capacity in mice alters host sensitivity to tumor necrosis factor toxicity but does not interfere with its antitumor efficacy. Cancer Res. 1990 Jun 15;50(12):3503–3508. [PubMed] [Google Scholar]
- Hirose K., Longo D. L., Oppenheim J. J., Matsushima K. Overexpression of mitochondrial manganese superoxide dismutase promotes the survival of tumor cells exposed to interleukin-1, tumor necrosis factor, selected anticancer drugs, and ionizing radiation. FASEB J. 1993 Feb 1;7(2):361–368. doi: 10.1096/fasebj.7.2.8440412. [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Yaginuma Y., Hayashi H., Shimizu T., Endo Y., Taniguchi N. Reactivity of a monoclonal antibody to manganese superoxide dismutase with human ovarian carcinoma. Cancer Res. 1990 Apr 15;50(8):2538–2542. [PubMed] [Google Scholar]
- Jass J. R., Atkin W. S., Cuzick J., Bussey H. J., Morson B. C., Northover J. M., Todd I. P. The grading of rectal cancer: historical perspectives and a multivariate analysis of 447 cases. Histopathology. 1986 May;10(5):437–459. doi: 10.1111/j.1365-2559.1986.tb02497.x. [DOI] [PubMed] [Google Scholar]
- Kizaki M., Sakashita A., Karmakar A., Lin C. W., Koeffler H. P. Regulation of manganese superoxide dismutase and other antioxidant genes in normal and leukemic hematopoietic cells and their relationship to cytotoxicity by tumor necrosis factor. Blood. 1993 Aug 15;82(4):1142–1150. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landriscina M., Remiddi F., Ria F., Palazzotti B., De Leo M. E., Iacoangeli M., Rosselli R., Scerrati M., Galeotti T. The level of MnSOD is directly correlated with grade of brain tumours of neuroepithelial origin. Br J Cancer. 1996 Dec;74(12):1877–1885. doi: 10.1038/bjc.1996.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Molmenti E. P., Ziambaras T., Perlmutter D. H. Evidence for an acute phase response in human intestinal epithelial cells. J Biol Chem. 1993 Jul 5;268(19):14116–14124. [PubMed] [Google Scholar]
- Mulder T. P., Verspaget H. W., Janssens A. R., de Bruin P. A., Griffioen G., Lamers C. B. Neoplasia-related changes of two copper (Cu)/zinc (Zn) proteins in the human colon. Free Radic Biol Med. 1990;9(6):501–506. doi: 10.1016/0891-5849(90)90128-6. [DOI] [PubMed] [Google Scholar]
- Nakano T., Oka K., Taniguchi N. Manganese superoxide dismutase expression correlates with p53 status and local recurrence of cervical carcinoma treated with radiation therapy. Cancer Res. 1996 Jun 15;56(12):2771–2775. [PubMed] [Google Scholar]
- Oberley L. W., Buettner G. R. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979 Apr;39(4):1141–1149. [PubMed] [Google Scholar]
- Oberley T. D., Oberley L. W. Antioxidant enzyme levels in cancer. Histol Histopathol. 1997 Apr;12(2):525–535. [PubMed] [Google Scholar]
- Ofner D., Maier H., Riedmann B., Bammer T., Rumer A., Winde G., Böcker W., Jasani B., Schmid K. W. Immunohistochemical metallothionein expression in colorectal adenocarcinoma: correlation with tumour stage and patient survival. Virchows Arch. 1994;425(5):491–497. doi: 10.1007/BF00197552. [DOI] [PubMed] [Google Scholar]
- Petkau A. Role of superoxide dismutase in modification of radiation injury. Br J Cancer Suppl. 1987 Jun;8:87–95. [PMC free article] [PubMed] [Google Scholar]
- Ponz de Leon M., Sant M., Micheli A., Sacchetti C., Di Gregorio C., Fante R., Zanghieri G., Melotti G., Gatta G. Clinical and pathologic prognostic indicators in colorectal cancer. A population-based study. Cancer. 1992 Feb 1;69(3):626–635. doi: 10.1002/1097-0142(19920201)69:3<626::aid-cncr2820690305>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Qureshi A., Gorey T. F., Byrne P., Kay E., McKeever J., Hennessy T. P. Oxygen free radical activity in experimental colonic carcinoma. Br J Surg. 1994 Jul;81(7):1058–1059. doi: 10.1002/bjs.1800810745. [DOI] [PubMed] [Google Scholar]
- Salim A. S. Removing oxygen-derived free radicals delays hepatic metastases and prolongs survival in colonic cancer. A study in the rat. Oncology. 1992;49(1):58–62. doi: 10.1159/000227012. [DOI] [PubMed] [Google Scholar]
- Sangeetha P., Das U. N., Koratkar R., Suryaprabha P. Increase in free radical generation and lipid peroxidation following chemotherapy in patients with cancer. Free Radic Biol Med. 1990;8(1):15–19. doi: 10.1016/0891-5849(90)90139-a. [DOI] [PubMed] [Google Scholar]
- Satomi A., Murakami S., Hashimoto T., Ishida K., Matsuki M., Sonoda M. Significance of superoxide dismutase (SOD) in human colorectal cancer tissue: correlation with malignant intensity. J Gastroenterol. 1995 Apr;30(2):177–182. doi: 10.1007/BF02348662. [DOI] [PubMed] [Google Scholar]
- Schadendorf D., Zuberbier T., Diehl S., Schadendorf C., Czarnetzki B. M. Serum manganese superoxide dismutase is a new tumour marker for malignant melanoma. Melanoma Res. 1995 Oct;5(5):351–353. doi: 10.1097/00008390-199510000-00008. [DOI] [PubMed] [Google Scholar]
- Sinnige H. A., Mulder N. H. Colorectal carcinoma: an update. Neth J Med. 1991 Jun;38(5-6):217–228. [PubMed] [Google Scholar]
- Slater T. F. Free-radical mechanisms in tissue injury. Biochem J. 1984 Aug 15;222(1):1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano M., Kuroda M., Reynolds R., Oberley T. D., St Clair D. K. Expression of manganese superoxide dismutase reduces tumor control radiation dose: gene-radiotherapy. Cancer Res. 1995 Jun 15;55(12):2490–2493. [PubMed] [Google Scholar]
- Valentine J. F., Nick H. S. Acute-phase induction of manganese superoxide dismutase in intestinal epithelial cell lines. Gastroenterology. 1992 Sep;103(3):905–912. doi: 10.1016/0016-5085(92)90024-s. [DOI] [PubMed] [Google Scholar]
- Warner B. B., Stuart L., Gebb S., Wispé J. R. Redox regulation of manganese superoxide dismutase. Am J Physiol. 1996 Jul;271(1 Pt 1):L150–L158. doi: 10.1152/ajplung.1996.271.1.L150. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Elwell J. H., Oberley L. W., Goeddel D. V. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989 Sep 8;58(5):923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988 Nov 11;242(4880):941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- Yoshimi N., Sato S., Makita H., Wang A., Hirose Y., Tanaka T., Mori H. Expression of cytokines, TNF-alpha and IL-1 alpha, in MAM acetate and 1-hydroxyanthraquinone-induced colon carcinogenesis of rats. Carcinogenesis. 1994 Apr;15(4):783–785. doi: 10.1093/carcin/15.4.783. [DOI] [PubMed] [Google Scholar]
- Zyad A., Bénard J., Tursz T., Clarke R., Chouaib S. Resistance to TNF-alpha and adriamycin in the human breast cancer MCF-7 cell line: relationship to MDR1, MnSOD, and TNF gene expression. Cancer Res. 1994 Feb 1;54(3):825–831. [PubMed] [Google Scholar]
- van Triest B., van Groeningen C. J., Pinedo H. M. Current chemotherapeutic possibilities in the treatment of colorectal cancer. Eur J Cancer. 1995 Jul-Aug;31A(7-8):1193–1197. doi: 10.1016/0959-8049(95)00161-b. [DOI] [PubMed] [Google Scholar]


