Abstract
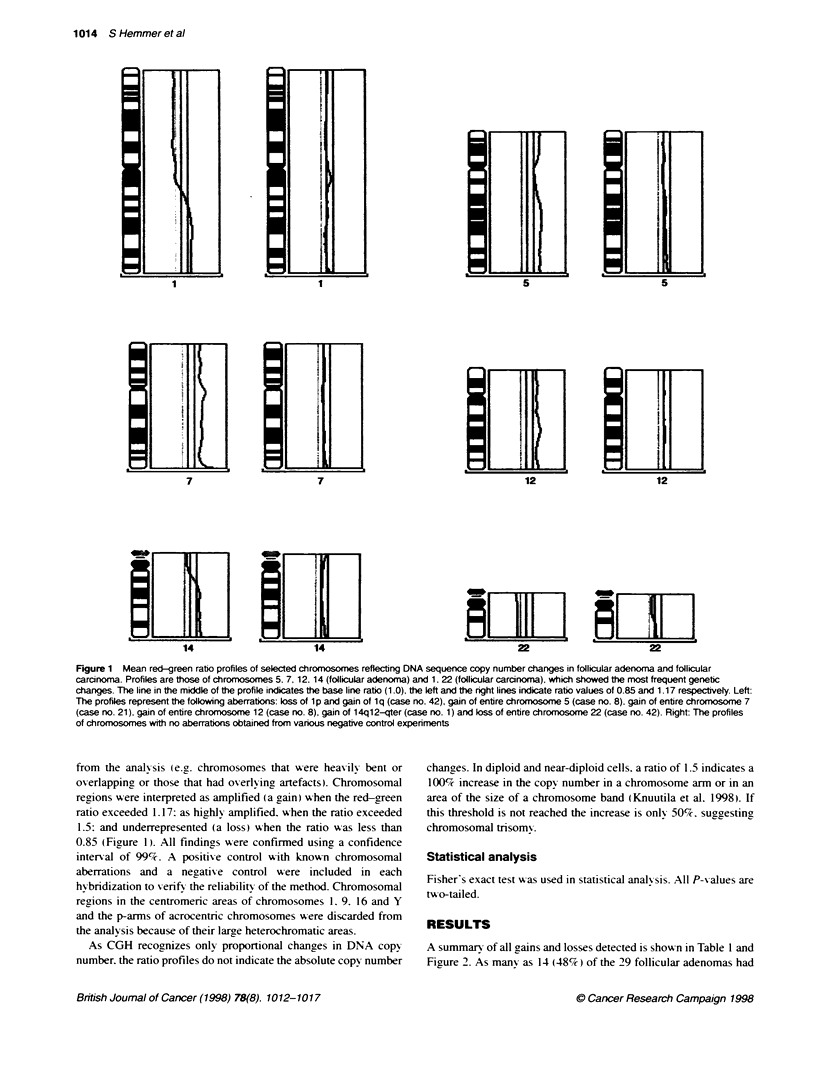
DNA copy number changes were compared in 29 histologically benign follicular adenomas, of which five were atypical, and 13 follicular carcinomas of the thyroid by comparative genomic hybridization. DNA copy number changes were frequent in adenomas (14 out of 29, 48%). Most changes were gains, and they always involved a gain of the entire chromosome 7 (10 out of 29, 34%); other common gains involved chromosomes 5 (28%), 9 (10%), 12 (24%), 14 (21%), 17 (17%), 18 (14%) and X (17%). Losses were found only in four (14%) adenomas. Two of the five atypical adenomas had DNA copy number losses, and none had gains. Unlike adenomas, gains were rare and losses were frequent in carcinomas. A loss of chromosome 22 or 22q was particularly common in carcinomas (6 out of 13, 46%), whereas a loss of chromosome 22 was found in only two (7%) adenomas, one of which was atypical (P = 0.002). A loss of 1p was also frequent in carcinomas (31%), but gains of chromosomes 5, 7, 12, 14 or X that were common in adenomas were not found. Loss of chromosome 22 or 22q was present in six of the eight widely invasive follicular carcinomas, but in only one of the five minimally invasive carcinomas. We conclude that large DNA copy number changes are common in thyroid adenomas. These changes are strikingly different from those found in follicular carcinomas consisting of few losses and frequent gains, especially those of chromosome 7. A loss of chromosome 22 is common in widely invasive follicular carcinoma.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belge G., Thode B., Rippe V., Bartnitzke S., Bullerdiek J. A characteristic sequence of trisomies starting with trisomy 7 in benign thyroid tumors. Hum Genet. 1994 Aug;94(2):198–202. doi: 10.1007/BF00202871. [DOI] [PubMed] [Google Scholar]
- Björkqvist A. M., Tammilehto L., Anttila S., Mattson K., Knuutila S. Recurrent DNA copy number changes in 1q, 4q, 6q, 9p, 13q, 14q and 22q detected by comparative genomic hybridization in malignant mesothelioma. Br J Cancer. 1997;75(4):523–527. doi: 10.1038/bjc.1997.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondeson L., Bengtsson A., Bondeson A. G., Dahlenfors R., Grimelius L., Wedell B., Mark J. Chromosome studies in thyroid neoplasia. Cancer. 1989 Aug 1;64(3):680–685. doi: 10.1002/1097-0142(19890801)64:3<680::aid-cncr2820640319>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Criado B., Barros A., Suijkerbuijk R. F., Weghuis D. O., Seruca R., Fonseca E., Castedo S. Detection of numerical alterations for chromosomes 7 and 12 in benign thyroid lesions by in situ hybridization. Histological implications. Am J Pathol. 1995 Jul;147(1):136–144. [PMC free article] [PubMed] [Google Scholar]
- Grebe S. K., McIver B., Hay I. D., Wu P. S., Maciel L. M., Drabkin H. A., Goellner J. R., Grant C. S., Jenkins R. B., Eberhardt N. L. Frequent loss of heterozygosity on chromosomes 3p and 17p without VHL or p53 mutations suggests involvement of unidentified tumor suppressor genes in follicular thyroid carcinoma. J Clin Endocrinol Metab. 1997 Nov;82(11):3684–3691. doi: 10.1210/jcem.82.11.4352. [DOI] [PubMed] [Google Scholar]
- Hedinger C., Williams E. D., Sobin L. H. The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer. 1989 Mar 1;63(5):908–911. doi: 10.1002/1097-0142(19890301)63:5<908::aid-cncr2820630520>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Herrmann M. A., Hay I. D., Bartelt D. H., Jr, Ritland S. R., Dahl R. J., Grant C. S., Jenkins R. B. Cytogenetic and molecular genetic studies of follicular and papillary thyroid cancers. J Clin Invest. 1991 Nov;88(5):1596–1604. doi: 10.1172/JCI115472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensuu H., Klemi P. J. DNA aneuploidy in adenomas of endocrine organs. Am J Pathol. 1988 Jul;132(1):145–151. [PMC free article] [PubMed] [Google Scholar]
- Knuutila S., Björkqvist A. M., Autio K., Tarkkanen M., Wolf M., Monni O., Szymanska J., Larramendy M. L., Tapper J., Pere H. DNA copy number amplifications in human neoplasms: review of comparative genomic hybridization studies. Am J Pathol. 1998 May;152(5):1107–1123. [PMC free article] [PubMed] [Google Scholar]
- Mohapatra G., Kim D. H., Feuerstein B. G. Detection of multiple gains and losses of genetic material in ten glioma cell lines by comparative genomic hybridization. Genes Chromosomes Cancer. 1995 Jun;13(2):86–93. doi: 10.1002/gcc.2870130203. [DOI] [PubMed] [Google Scholar]
- Roque L., Castedo S., Clode A., Soares J. Deletion of 3p25-->pter in a primary follicular thyroid carcinoma and its metastasis. Genes Chromosomes Cancer. 1993 Nov;8(3):199–203. doi: 10.1002/gcc.2870080311. [DOI] [PubMed] [Google Scholar]
- Roque L., Gomes P., Correia C., Soares P., Soares J., Castedo S. Thyroid nodular hyperplasia: chromosomal studies in 14 cases. Cancer Genet Cytogenet. 1993 Aug;69(1):31–34. doi: 10.1016/0165-4608(93)90108-x. [DOI] [PubMed] [Google Scholar]
- Ruttledge M. H., Sarrazin J., Rangaratnam S., Phelan C. M., Twist E., Merel P., Delattre O., Thomas G., Nordenskjöld M., Collins V. P. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat Genet. 1994 Feb;6(2):180–184. doi: 10.1038/ng0294-180. [DOI] [PubMed] [Google Scholar]
- Schofield D. E., Beckwith J. B., Sklar J. Loss of heterozygosity at chromosome regions 22q11-12 and 11p15.5 in renal rhabdoid tumors. Genes Chromosomes Cancer. 1996 Jan;15(1):10–17. doi: 10.1002/(SICI)1098-2264(199601)15:1<10::AID-GCC2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Teyssier J. R., Liautaud-Roger F., Ferre D., Patey M., Dufer J. Chromosomal changes in thyroid tumors. Relation with DNA content, karyotypic features, and clinical data. Cancer Genet Cytogenet. 1990 Dec;50(2):249–263. doi: 10.1016/0165-4608(90)90184-c. [DOI] [PubMed] [Google Scholar]
- Tonk V., Osella P., Delasmorenas A., Wyandt H. E., Milunsky A. Abnormalities of chromosome 22 in meningiomas and confirmation of the origin of a dicentric 22 by in situ hybridization. Cancer Genet Cytogenet. 1992 Nov;64(1):65–68. doi: 10.1016/0165-4608(92)90325-3. [DOI] [PubMed] [Google Scholar]
- WOOLNER L. B., BEAHRS O. H., BLACK B. M., McCONAHEY W. M., KEATING F. R., Jr Classification and prognosis of thyroid carcinoma. A study of 885 cases observed in a thirty year period. Am J Surg. 1961 Sep;102:354–387. doi: 10.1016/0002-9610(61)90527-x. [DOI] [PubMed] [Google Scholar]
- el-Rifai W., Larramendy M. L., Björkqvist A. M., Hemmer S., Knuutila S. Optimization of comparative genomic hybridization using fluorochrome conjugated to dCTP and dUTP nucleotides. Lab Invest. 1997 Dec;77(6):699–700. [PubMed] [Google Scholar]
- van den Berg E., Oosterhuis J. W., de Jong B., Buist J., Vos A., Dam A., Vermeij B. Cytogenetics of thyroid follicular adenomas. Cancer Genet Cytogenet. 1990 Feb;44(2):217–222. doi: 10.1016/0165-4608(90)90050-k. [DOI] [PubMed] [Google Scholar]
- van den Berg E., van Doormaal J. J., Oosterhuis J. W., de Jong B., Wiersema J., Vos A. M., Dam A., Vermeij A. Chromosomal aberrations in follicular thyroid carcinoma. Case report of a primary tumor and its metastasis. Cancer Genet Cytogenet. 1991 Jul 15;54(2):215–222. doi: 10.1016/0165-4608(91)90209-d. [DOI] [PubMed] [Google Scholar]


