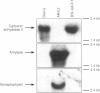
Abstract
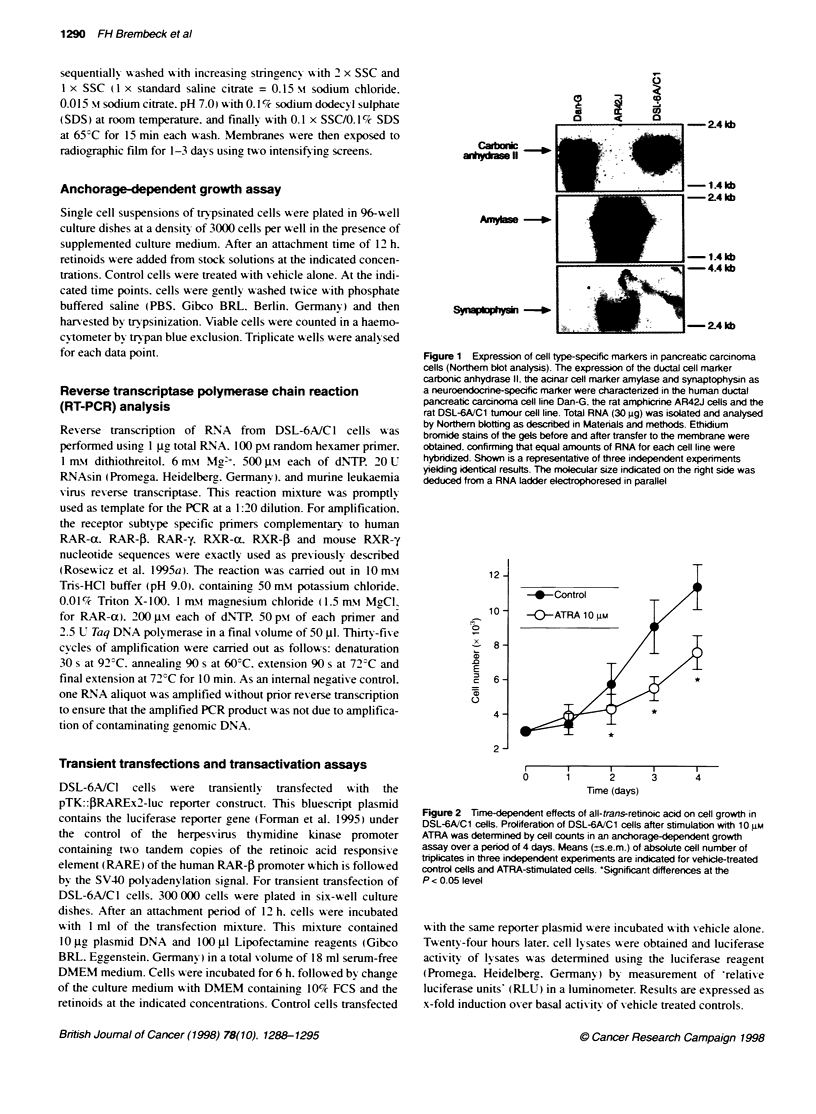
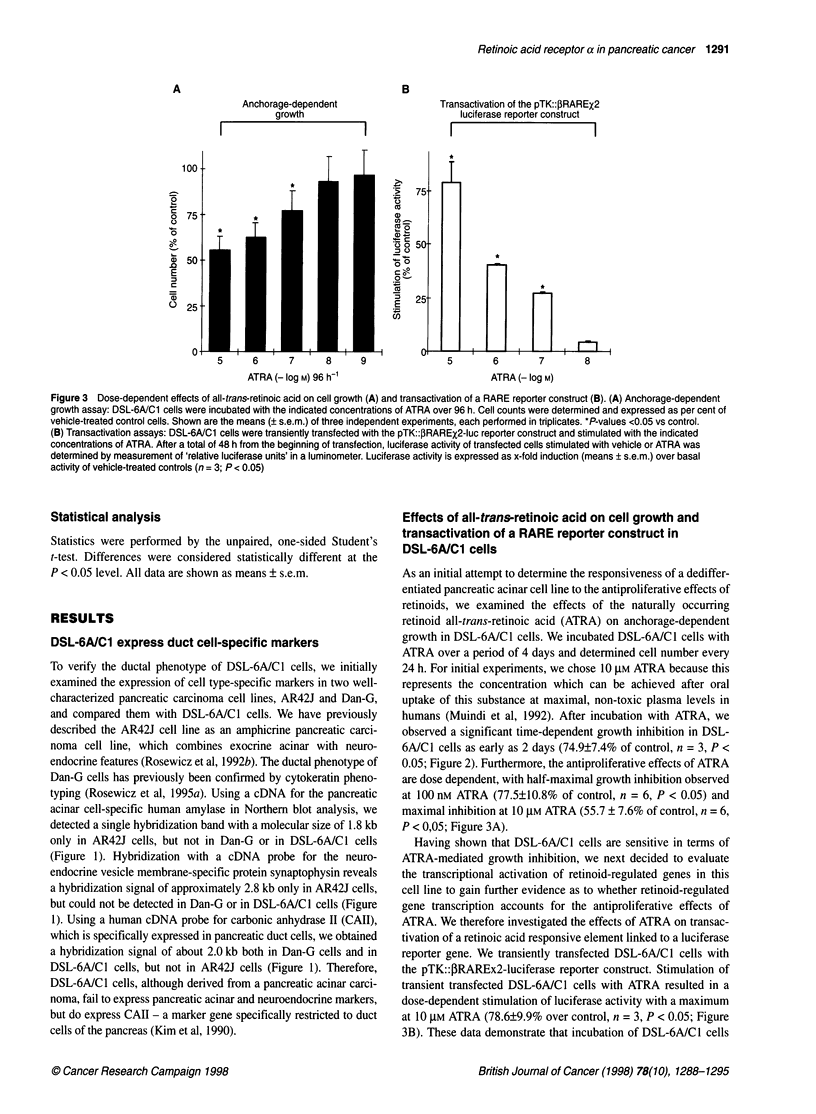
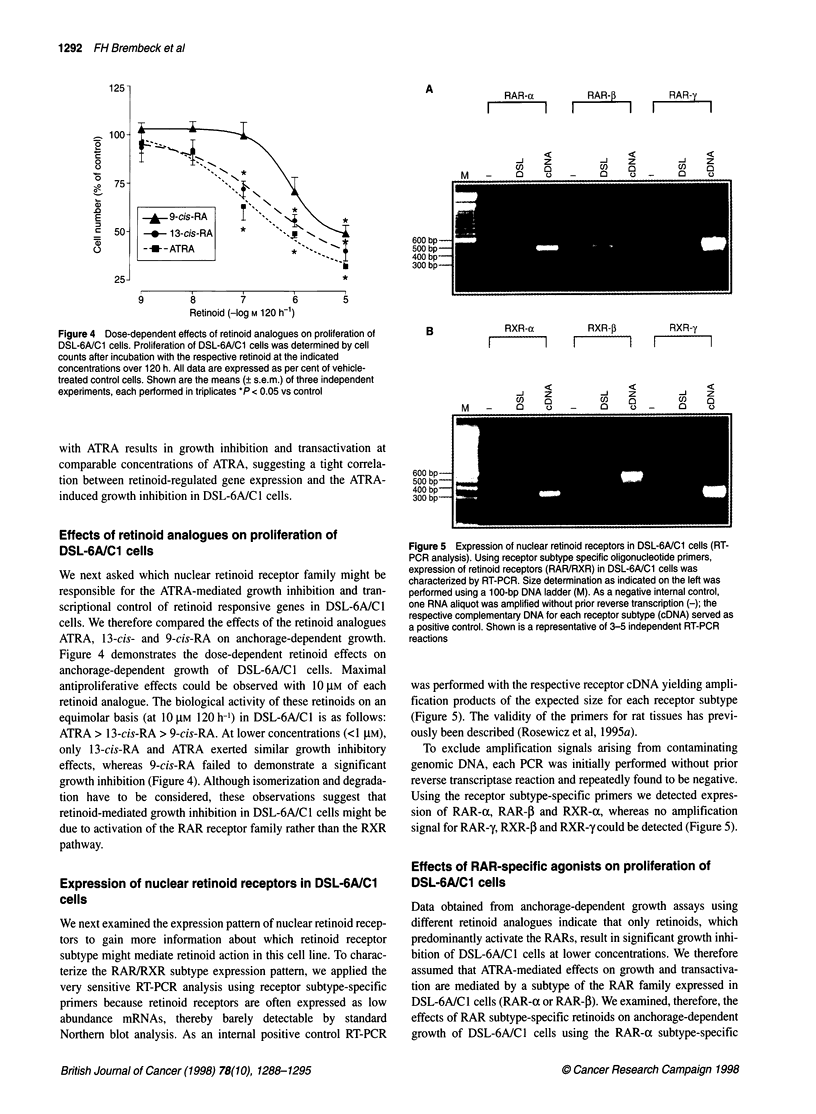
During carcinogenesis, pancreatic acinar cells can dedifferentiate into ductal adenocarcinoma of the pancreas. DSL-6A/C1 cells represent an in vitro model of this carcinogenic sequence. This study was designed to examine the effects of retinoids on cell growth in DSL-6A/C1 cells and to characterize further the molecular mechanisms underlying the antiproliferative actions of retinoids. Treatment of DSL-6A/C1 cells with retinoids results in a time- and dose-dependent inhibition of cell growth, paralleled by a retinoid-mediated transactivation of a pTK::betaRAREx2-luciferase reporter construct transiently transfected into DSL-6A/C1 cells. Retinoid receptor expression was evaluated by reverse transcriptase polymerase chain reaction (RT-PCR) using subtype-specific primers and demonstrated expression of retinoic acid receptor alpha (RAR-alpha), RAR-beta and retinoid X receptor alpha (RXR-alpha). Using a panel of receptor subtype-specific agonists, the RAR-alpha specific agonist Ro 40-6055 was the most potent retinoid in terms of growth inhibition. Furthermore, all-trans-retinoic acid-mediated growth inhibition and transactivation was completely blocked by the RAR-alpha-specific antagonist Ro 41-5253. In summary, the RAR-alpha subtype predominantly mediates the antiproliferative effects of retinoids in DSL-6A/C1 cells. Furthermore, this cell system provides a feasible tool to study the molecular mechanisms underlying the growth inhibitory effects of retinoids in ductal pancreatic carcinoma cells derived from a primary acinar cell phenotype.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apfel C., Bauer F., Crettaz M., Forni L., Kamber M., Kaufmann F., LeMotte P., Pirson W., Klaus M. A retinoic acid receptor alpha antagonist selectively counteracts retinoic acid effects. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7129–7133. doi: 10.1073/pnas.89.15.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockman D. E., Black O., Jr, Mills L. R., Mainz D. L., Webster P. D., 3rd Fine structure of pancreatic adenocarcinoma induced in rats by 7,12-dimethylbenz(a)anthracene. J Natl Cancer Inst. 1976 Oct;57(4):931–936. doi: 10.1093/jnci/57.4.931. [DOI] [PubMed] [Google Scholar]
- Bockman D. E., Black O., Jr, Mills L. R., Webster P. D. Origin of tubular complexes developing during induction of pancreatic adenocarcinoma by 7,12-dimethylbenz(a)anthracene. Am J Pathol. 1978 Mar;90(3):645–658. [PMC free article] [PubMed] [Google Scholar]
- Bollag W., Holdener E. E. Retinoids in cancer prevention and therapy. Ann Oncol. 1992 Jul;3(7):513–526. doi: 10.1093/oxfordjournals.annonc.a058252. [DOI] [PubMed] [Google Scholar]
- Cerny W. L., Mangold K. A., Scarpelli D. G. Activation of K-ras in transplantable pancreatic ductal adenocarcinomas of Syrian golden hamsters. Carcinogenesis. 1990 Nov;11(11):2075–2079. doi: 10.1093/carcin/11.11.2075. [DOI] [PubMed] [Google Scholar]
- Crettaz M., Baron A., Siegenthaler G., Hunziker W. Ligand specificities of recombinant retinoic acid receptors RAR alpha and RAR beta. Biochem J. 1990 Dec 1;272(2):391–397. doi: 10.1042/bj2720391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lisle R. C., Logsdon C. D. Pancreatic acinar cells in culture: expression of acinar and ductal antigens in a growth-related manner. Eur J Cell Biol. 1990 Feb;51(1):64–75. [PubMed] [Google Scholar]
- Dissin J., Mills L. R., Mains D. L., Black O., Jr, Webster P. D., 3rd Experimental induction of pancreatic adenocarcinoma in rats. J Natl Cancer Inst. 1975 Oct;55(4):857–864. doi: 10.1093/jnci/55.4.857. [DOI] [PubMed] [Google Scholar]
- Dollé P., Ruberte E., Leroy P., Morriss-Kay G., Chambon P. Retinoic acid receptors and cellular retinoid binding proteins. I. A systematic study of their differential pattern of transcription during mouse organogenesis. Development. 1990 Dec;110(4):1133–1151. doi: 10.1242/dev.110.4.1133. [DOI] [PubMed] [Google Scholar]
- Durand B., Saunders M., Leroy P., Leid M., Chambon P. All-trans and 9-cis retinoic acid induction of CRABPII transcription is mediated by RAR-RXR heterodimers bound to DR1 and DR2 repeated motifs. Cell. 1992 Oct 2;71(1):73–85. doi: 10.1016/0092-8674(92)90267-g. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Umesono K., Chen J., Evans R. M. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995 May 19;81(4):541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Giguère V. Retinoic acid receptors and cellular retinoid binding proteins: complex interplay in retinoid signaling. Endocr Rev. 1994 Feb;15(1):61–79. doi: 10.1210/edrv-15-1-61. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988 Nov;4(11):309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Kastner P., Krust A., Mendelsohn C., Garnier J. M., Zelent A., Leroy P., Staub A., Chambon P. Murine isoforms of retinoic acid receptor gamma with specific patterns of expression. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2700–2704. doi: 10.1073/pnas.87.7.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Ho S. B., Montgomery C. K., Kim Y. S. Cell lineage markers in human pancreatic cancer. Cancer. 1990 Nov 15;66(10):2134–2143. doi: 10.1002/1097-0142(19901115)66:10<2134::aid-cncr2820661016>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee X., Si S. P., Tsou H. C., Peacocke M. Cellular aging and transformation suppression: a role for retinoic acid receptor beta 2. Exp Cell Res. 1995 May;218(1):296–304. doi: 10.1006/excr.1995.1158. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci. 1992 Oct;17(10):427–433. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Lionetto R., Pugliese V., Bruzzi P., Rosso R. No standard treatment is available for advanced pancreatic cancer. Eur J Cancer. 1995 Jun;31A(6):882–887. doi: 10.1016/0959-8049(94)00445-5. [DOI] [PubMed] [Google Scholar]
- Longnecker D. S., Curphey T. J. Adenocarcinoma of the pancreas in azaserine-treated rats. Cancer Res. 1975 Aug;35(8):2249–2258. [PubMed] [Google Scholar]
- Longnecker D. S., Memoli V., Pettengill O. S. Recent results in animal models of pancreatic carcinoma: histogenesis of tumors. Yale J Biol Med. 1992 Sep-Oct;65(5):457–469. [PMC free article] [PubMed] [Google Scholar]
- Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1980 Mar 12;605(1):33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Lotan R., Lotan D., Sacks P. G. Inhibition of tumor cell growth by retinoids. Methods Enzymol. 1990;190:100–110. doi: 10.1016/0076-6879(90)90014-r. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Borgmeyer U., Heyman R. A., Zhou J. Y., Ong E. S., Oro A. E., Kakizuka A., Evans R. M. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992 Mar;6(3):329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Evans R. M. The RXR heterodimers and orphan receptors. Cell. 1995 Dec 15;83(6):841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- Marshall G. M., Cheung B., Stacey K. P., Camacho M. L., Simpson A. M., Kwan E., Smith S., Haber M., Norris M. D. Increased retinoic acid receptor gamma expression suppresses the malignant phenotype and alters the differentiation potential of human neuroblastoma cells. Oncogene. 1995 Aug 3;11(3):485–491. [PubMed] [Google Scholar]
- Moasser M. M., DeBlasio A., Dmitrovsky E. Response and resistance to retinoic acid are mediated through the retinoic acid nuclear receptor gamma in human teratocarcinomas. Oncogene. 1994 Mar;9(3):833–840. [PubMed] [Google Scholar]
- Muindi J. R., Frankel S. R., Huselton C., DeGrazia F., Garland W. A., Young C. W., Warrell R. P., Jr Clinical pharmacology of oral all-trans retinoic acid in patients with acute promyelocytic leukemia. Cancer Res. 1992 Apr 15;52(8):2138–2142. [PubMed] [Google Scholar]
- När A. M., Boutin J. M., Lipkin S. M., Yu V. C., Holloway J. M., Glass C. K., Rosenfeld M. G. The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell. 1991 Jun 28;65(7):1267–1279. doi: 10.1016/0092-8674(91)90021-p. [DOI] [PubMed] [Google Scholar]
- Pettengill O. S., Faris R. A., Bell R. H., Jr, Kuhlmann E. T., Longnecker D. S. Derivation of ductlike cell lines from a transplantable acinar cell carcinoma of the rat pancreas. Am J Pathol. 1993 Jul;143(1):292–303. [PMC free article] [PubMed] [Google Scholar]
- Rosewicz S., Detjen K., Kaiser A., Prosenc N., Cervos-Navarro J., Riecken E. O., Haller H. Bombesin receptor gene expression in rat pancreatic acinar AR42J cells: transcriptional regulation by glucocorticoids. Gastroenterology. 1994 Jul;107(1):208–218. doi: 10.1016/0016-5085(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Rosewicz S., Lewis L. D., Wang X. Y., Liddle R. A., Logsdon C. D. Pancreatic digestive enzyme gene expression: effects of CCK and soybean trypsin inhibitor. Am J Physiol. 1989 Apr;256(4 Pt 1):G733–G738. doi: 10.1152/ajpgi.1989.256.4.G733. [DOI] [PubMed] [Google Scholar]
- Rosewicz S., Riecken E. O., Stier U. Transcriptional regulation of carbonic anhydrase II by retinoic acid in the human pancreatic tumor cell line DANG. FEBS Lett. 1995 Jul 10;368(1):45–48. doi: 10.1016/0014-5793(95)00596-2. [DOI] [PubMed] [Google Scholar]
- Rosewicz S., Riecken E. O., Wiedenmann B. The amphicrine pancreatic cell line AR42J: a model system for combined studies on exocrine and endocrine secretion. Clin Investig. 1992 Mar-Apr;70(3-4):205–209. doi: 10.1007/BF00184652. [DOI] [PubMed] [Google Scholar]
- Rosewicz S., Stier U., Brembeck F., Kaiser A., Papadimitriou C. A., Berdel W. E., Wiedenmann B., Riecken E. O. Retinoids: effects on growth, differentiation, and nuclear receptor expression in human pancreatic carcinoma cell lines. Gastroenterology. 1995 Nov;109(5):1646–1660. doi: 10.1016/0016-5085(95)90655-x. [DOI] [PubMed] [Google Scholar]
- Rosewicz S., Vogt D., Harth N., Grund C., Franke W. W., Ruppert S., Schweitzer E., Riecken E. O., Wiedenmann B. An amphicrine pancreatic cell line: AR42J cells combine exocrine and neuroendocrine properties. Eur J Cell Biol. 1992 Oct;59(1):80–91. [PubMed] [Google Scholar]
- Satake K., Mukai R., Kato Y., Shim K., Umeyama K. Experimental pancreatic carcinoma as a model of human pancreatic carcinoma. Clin Oncol. 1984 Mar;10(1):27–34. [PubMed] [Google Scholar]
- Scarpelli D. G., Rao M. S., Reddy J. K. Studies of pancreatic carcinogenesis in different animal models. Environ Health Perspect. 1984 Jun;56:219–227. [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Parkinson D. R., Cheson B. D., Friedman M. A. Retinoids in cancer therapy. J Clin Oncol. 1992 May;10(5):839–864. doi: 10.1200/JCO.1992.10.5.839. [DOI] [PubMed] [Google Scholar]
- Tallman M. S., Wiernik P. H. Retinoids in cancer treatment. J Clin Pharmacol. 1992 Oct;32(10):868–888. doi: 10.1002/j.1552-4604.1992.tb04633.x. [DOI] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelent A., Mendelsohn C., Kastner P., Krust A., Garnier J. M., Ruffenach F., Leroy P., Chambon P. Differentially expressed isoforms of the mouse retinoic acid receptor beta generated by usage of two promoters and alternative splicing. EMBO J. 1991 Jan;10(1):71–81. doi: 10.1002/j.1460-2075.1991.tb07922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Leede B. J., Folkers G. E., van den Brink C. E., van der Saag P. T., van der Burg B. Retinoic acid receptor alpha 1 isoform is induced by estradiol and confers retinoic acid sensitivity in human breast cancer cells. Mol Cell Endocrinol. 1995 Mar;109(1):77–86. doi: 10.1016/0303-7207(95)03487-r. [DOI] [PubMed] [Google Scholar]