Abstract
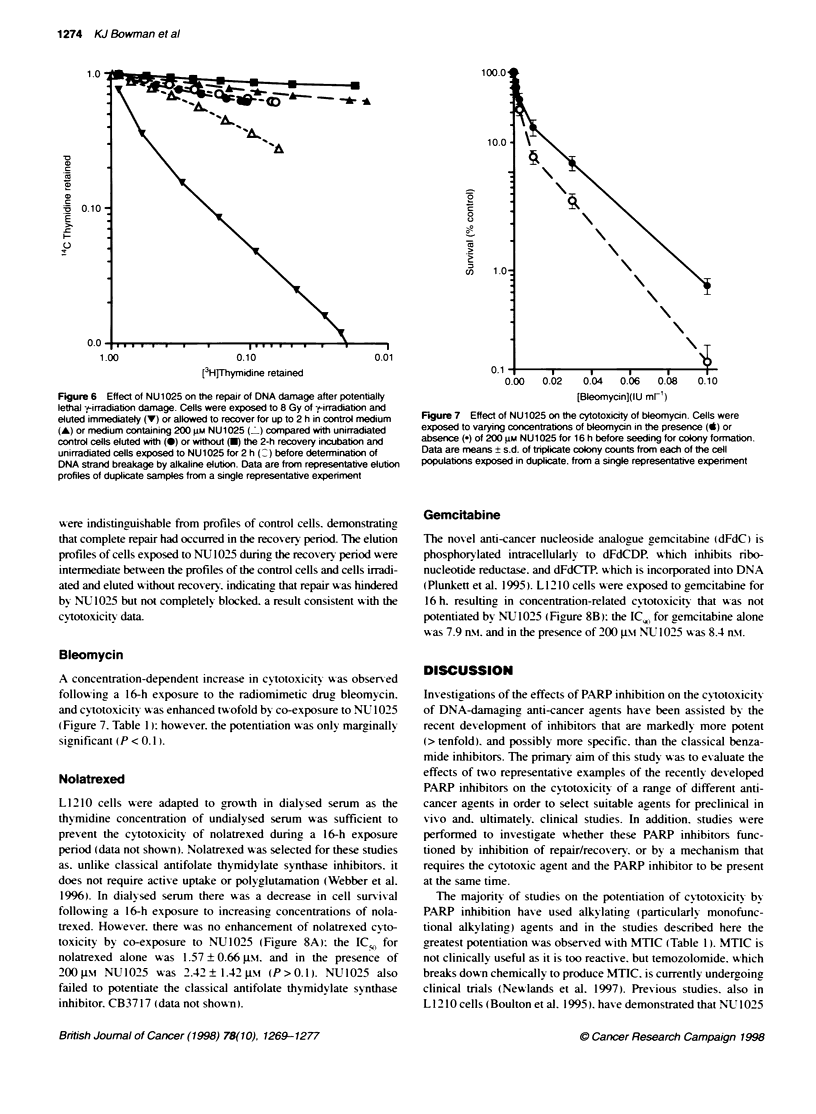
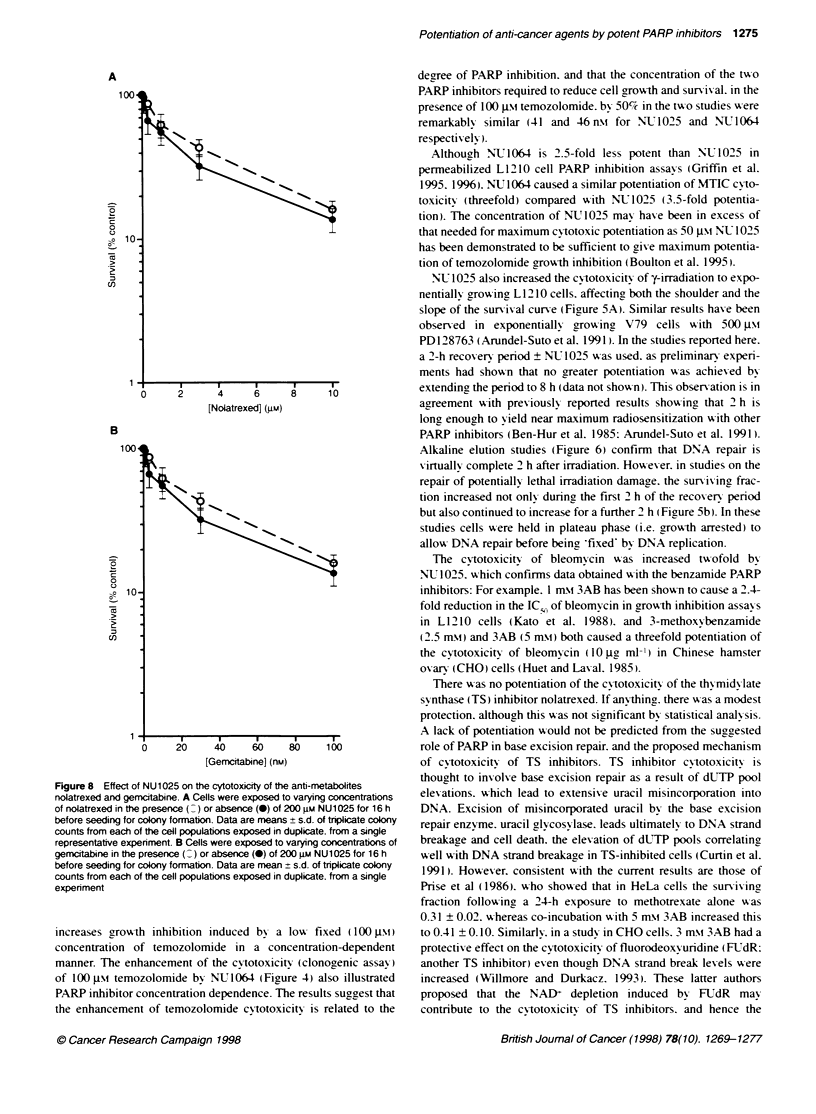
The ability of the potent poly(ADP-ribose) polymerase (PARP) inhibitor, NU1025 (8-hydroxy-2-methyl-quinazolin-4-[3H]one) to potentiate the cytotoxicity of a panel of mechanistically diverse anti-cancer agents was evaluated in L1210 cells. NU1025 enhanced the cytotoxicity of the DNA-methylating agent MTIC, gamma-irradiation and bleomycin 3.5-, 1.4- and 2-fold respectively. The cytotoxicities of the thymidylate synthase inhibitor, nolatrexed, and the cytotoxic nucleoside, gemcitabine, were not increased. Potentiation of MTIC cytotoxicity by a delayed exposure to NU1025 was equally effective as by a simultaneous exposure to NU1025, indicating that the effects of NU1025 were mediated by an inhibition of the cellular recovery. The recovery from potentially lethal gamma-irradiation damage cytotoxicity in plateau-phase cells was also inhibited by NU1025. Investigation of DNA strand breakage and repair in gamma-irradiated cells by alkaline elution demonstrated that NU1025 caused a marked retardation of DNA repair. A structurally different PARP inhibitor, NU1064 (2-methylbenzimidazole-4-carboxamide), also potentiated the cytotoxicity of MTIC, to a similar extent to NU1025. NU1064 potentiated a sublethal concentration of a DNA methylating agent in a concentration-dependent manner. Collectively, these data suggest that the most suitable cytotoxic agents for use in combination with PARP inhibitors are methylating agents, bleomycin and ionizing radiation, but not anti-metabolites.
Full text
PDF
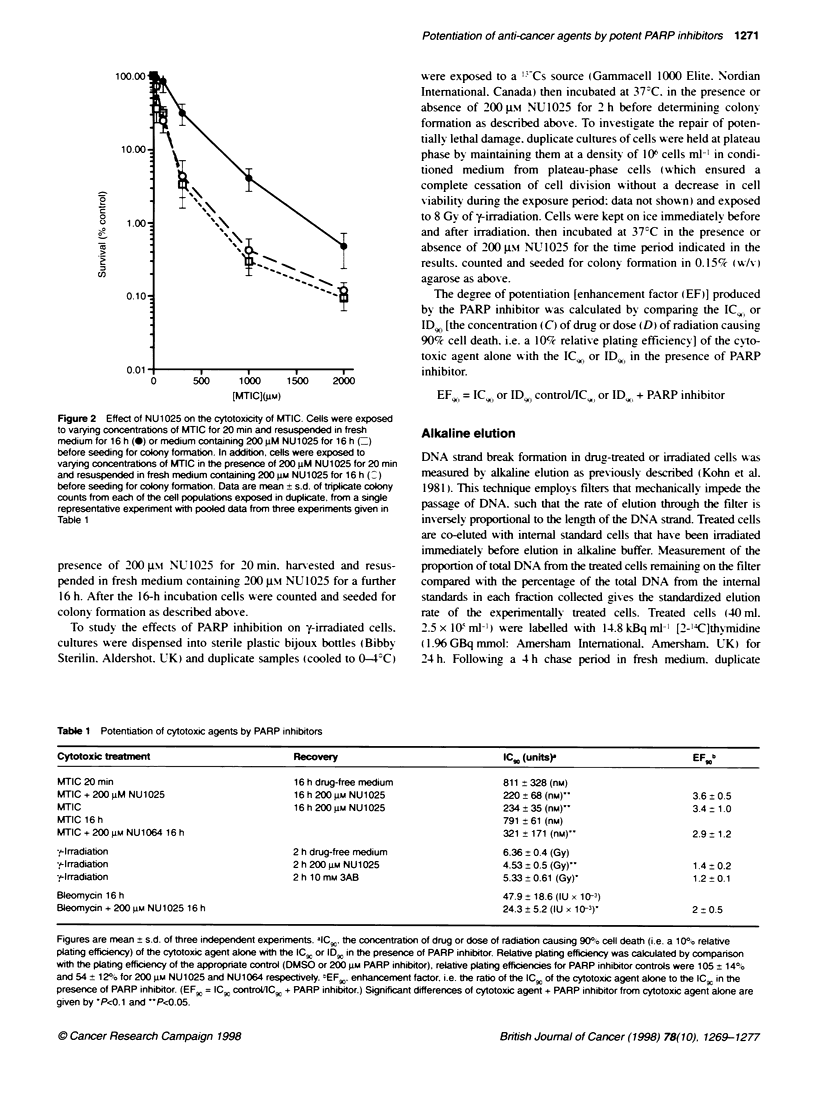
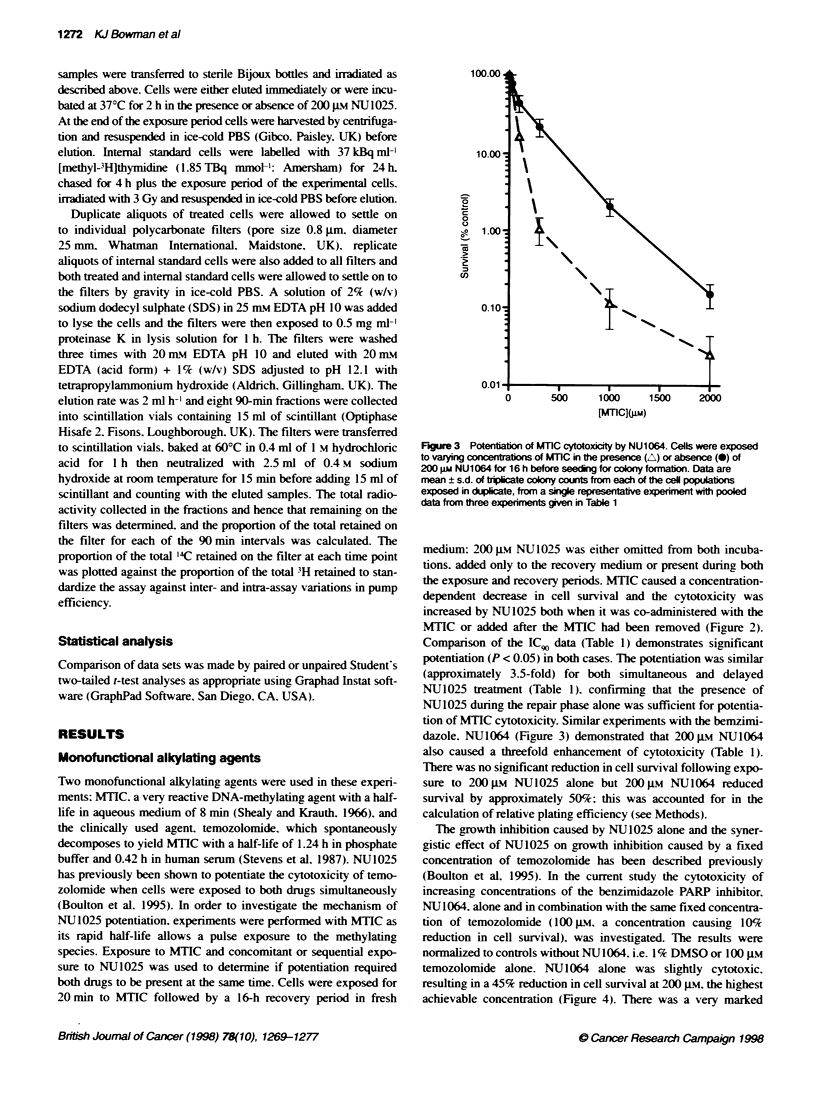
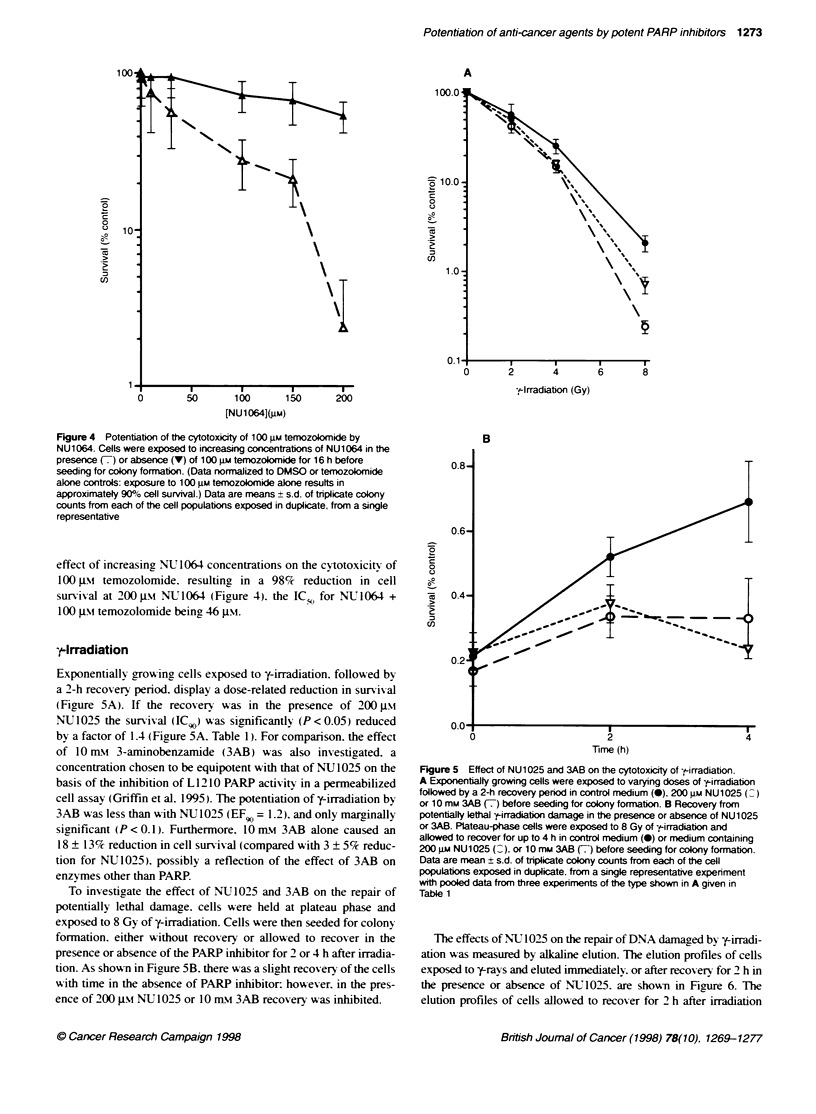


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althaus F. R., Höfferer L., Kleczkowska H. E., Malanga M., Naegeli H., Panzeter P., Realini C. Histone shuttle driven by the automodification cycle of poly(ADP-ribose)polymerase. Environ Mol Mutagen. 1993;22(4):278–282. doi: 10.1002/em.2850220417. [DOI] [PubMed] [Google Scholar]
- Arundel-Suto C. M., Scavone S. V., Turner W. R., Suto M. J., Sebolt-Leopold J. S. Effect of PD 128763, a new potent inhibitor of poly(ADP-ribose) polymerase, on X-ray-induced cellular recovery processes in Chinese hamster V79 cells. Radiat Res. 1991 Jun;126(3):367–371. [PubMed] [Google Scholar]
- Ben-Hur E., Chen C. C., Elkind M. M. Inhibitors of poly(adenosine diphosphoribose) synthetase, examination of metabolic perturbations, and enhancement of radiation response in Chinese hamster cells. Cancer Res. 1985 May;45(5):2123–2127. [PubMed] [Google Scholar]
- Boulikas T. Relation between carcinogenesis, chromatin structure and poly(ADP-ribosylation) (review). Anticancer Res. 1991 Mar-Apr;11(2):489–527. [PubMed] [Google Scholar]
- Boulton S., Pemberton L. C., Porteous J. K., Curtin N. J., Griffin R. J., Golding B. T., Durkacz B. W. Potentiation of temozolomide-induced cytotoxicity: a comparative study of the biological effects of poly(ADP-ribose) polymerase inhibitors. Br J Cancer. 1995 Oct;72(4):849–856. doi: 10.1038/bjc.1995.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Morgan W. F. Poly(ADP-ribose)polymerase: a perplexing participant in cellular responses to DNA breakage. Mutat Res. 1991 Jan;257(1):1–18. doi: 10.1016/0165-1110(91)90016-o. [DOI] [PubMed] [Google Scholar]
- Huang P., Plunkett W. Fludarabine- and gemcitabine-induced apoptosis: incorporation of analogs into DNA is a critical event. Cancer Chemother Pharmacol. 1995;36(3):181–188. doi: 10.1007/BF00685844. [DOI] [PubMed] [Google Scholar]
- Huet J., Laval F. Potentiation of cell killing by inhibitors of poly(adenosine diphosphate-ribose) synthesis in bleomycin-treated Chinese hamster ovary cells. Cancer Res. 1985 Mar;45(3):987–991. [PubMed] [Google Scholar]
- Hunting D. J., Gowans B. J., Henderson J. F. Specificity of inhibitors of poly(ADP-ribose) synthesis. Effects on nucleotide metabolism in cultured cells. Mol Pharmacol. 1985 Aug;28(2):200–206. [PubMed] [Google Scholar]
- Kato T., Suzumura Y., Fukushima M. Enhancement of bleomycin activity by 3-aminobenzamide, a poly (ADP-ribose) synthesis inhibitor, in vitro and in vivo. Anticancer Res. 1988 Mar-Apr;8(2):239–243. [PubMed] [Google Scholar]
- Kaufmann S. H., Desnoyers S., Ottaviano Y., Davidson N. E., Poirier G. G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993 Sep 1;53(17):3976–3985. [PubMed] [Google Scholar]
- Küpper J. H., van Gool L., Bürkle A. Molecular genetic systems to study the role of poly(ADP-ribosyl)ation in the cellular response to DNA damage. Biochimie. 1995;77(6):450–455. doi: 10.1016/0300-9084(96)88159-4. [DOI] [PubMed] [Google Scholar]
- Lautier D., Lagueux J., Thibodeau J., Ménard L., Poirier G. G. Molecular and biochemical features of poly (ADP-ribose) metabolism. Mol Cell Biochem. 1993 May 26;122(2):171–193. doi: 10.1007/BF01076101. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Satoh M. S., Poirier G. G., Klungland A. Post-translational modification of poly(ADP-ribose) polymerase induced by DNA strand breaks. Trends Biochem Sci. 1995 Oct;20(10):405–411. doi: 10.1016/s0968-0004(00)89089-1. [DOI] [PubMed] [Google Scholar]
- Milam K. M., Thomas G. H., Cleaver J. E. Disturbances in DNA precursor metabolism associated with exposure to an inhibitor of poly(ADP-ribose) synthetase. Exp Cell Res. 1986 Jul;165(1):260–268. doi: 10.1016/0014-4827(86)90550-1. [DOI] [PubMed] [Google Scholar]
- Molinete M., Vermeulen W., Bürkle A., Ménissier-de Murcia J., Küpper J. H., Hoeijmakers J. H., de Murcia G. Overproduction of the poly(ADP-ribose) polymerase DNA-binding domain blocks alkylation-induced DNA repair synthesis in mammalian cells. EMBO J. 1993 May;12(5):2109–2117. doi: 10.1002/j.1460-2075.1993.tb05859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses K., Harris A. L., Durkacz B. W. Adenosine-diphosphoribosyltransferase inhibitors can protect against or potentiate the cytotoxicity of S-phase acting drugs. Biochem Pharmacol. 1988 Jun 1;37(11):2155–2160. doi: 10.1016/0006-2952(88)90575-8. [DOI] [PubMed] [Google Scholar]
- Newlands E. S., Stevens M. F., Wedge S. R., Wheelhouse R. T., Brock C. Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev. 1997 Jan;23(1):35–61. doi: 10.1016/s0305-7372(97)90019-0. [DOI] [PubMed] [Google Scholar]
- Plunkett W., Huang P., Xu Y. Z., Heinemann V., Grunewald R., Gandhi V. Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Oncol. 1995 Aug;22(4 Suppl 11):3–10. [PubMed] [Google Scholar]
- Prise K. M., Gaal J. C., Pearson C. K. Increased protein ADPribosylation in HeLa cells exposed to the anti-cancer drug methotrexate. Biochim Biophys Acta. 1986 Jun 16;887(1):13–22. doi: 10.1016/0167-4889(86)90116-3. [DOI] [PubMed] [Google Scholar]
- Satoh M. S., Lindahl T. Role of poly(ADP-ribose) formation in DNA repair. Nature. 1992 Mar 26;356(6367):356–358. doi: 10.1038/356356a0. [DOI] [PubMed] [Google Scholar]
- Stevens M. F., Hickman J. A., Langdon S. P., Chubb D., Vickers L., Stone R., Baig G., Goddard C., Gibson N. W., Slack J. A. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res. 1987 Nov 15;47(22):5846–5852. [PubMed] [Google Scholar]
- Wang Z. Q., Auer B., Stingl L., Berghammer H., Haidacher D., Schweiger M., Wagner E. F. Mice lacking ADPRT and poly(ADP-ribosyl)ation develop normally but are susceptible to skin disease. Genes Dev. 1995 Mar 1;9(5):509–520. doi: 10.1101/gad.9.5.509. [DOI] [PubMed] [Google Scholar]
- Webber S., Bartlett C. A., Boritzki T. J., Hillard J. A., Howland E. F., Johnston A. L., Kosa M., Margosiak S. A., Morse C. A., Shetty B. V. AG337, a novel lipophilic thymidylate synthase inhibitor: in vitro and in vivo preclinical studies. Cancer Chemother Pharmacol. 1996;37(6):509–517. doi: 10.1007/s002800050422. [DOI] [PubMed] [Google Scholar]
- Willmore E., Durkacz B. W. Cytotoxic mechanisms of 5-fluoropyrimidines. Relationships with poly(ADP-ribose) polymerase activity, DNA strand breakage and incorporation into nucleic acids. Biochem Pharmacol. 1993 Jul 20;46(2):205–211. doi: 10.1016/0006-2952(93)90405-l. [DOI] [PubMed] [Google Scholar]
- de Murcia G., Ménissier de Murcia J. Poly(ADP-ribose) polymerase: a molecular nick-sensor. Trends Biochem Sci. 1994 Apr;19(4):172–176. doi: 10.1016/0968-0004(94)90280-1. [DOI] [PubMed] [Google Scholar]


