Abstract
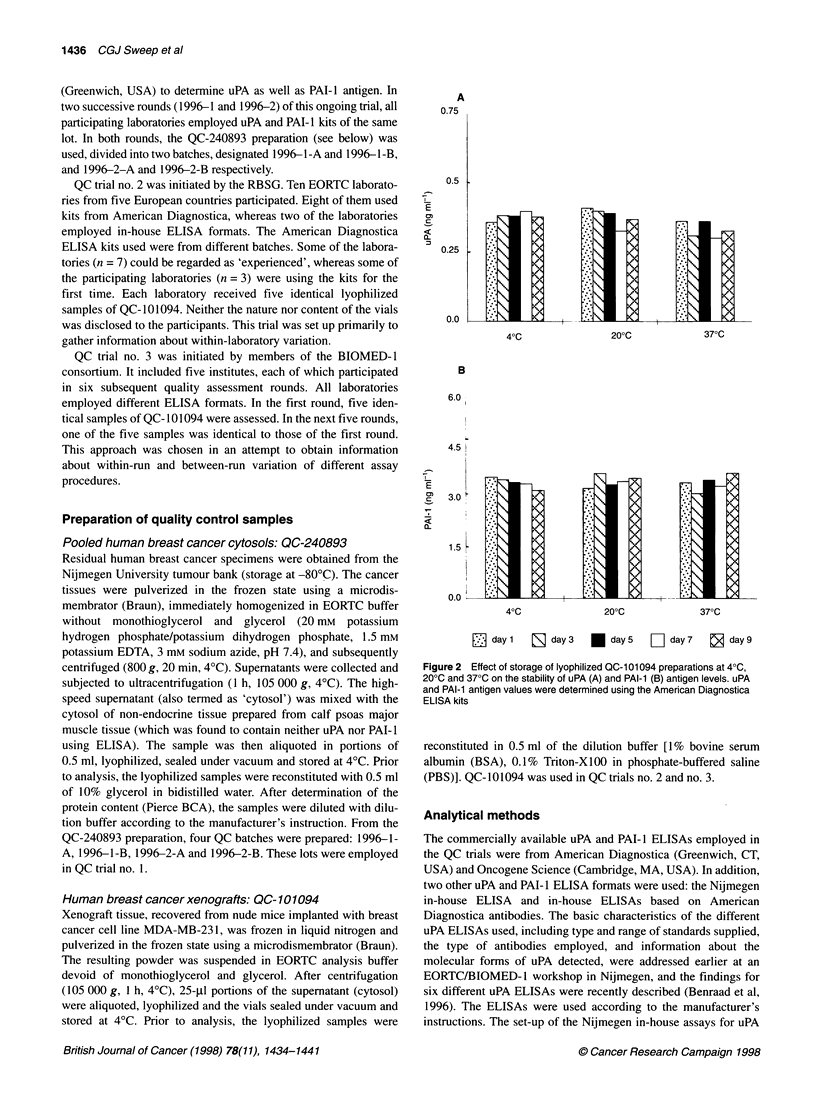
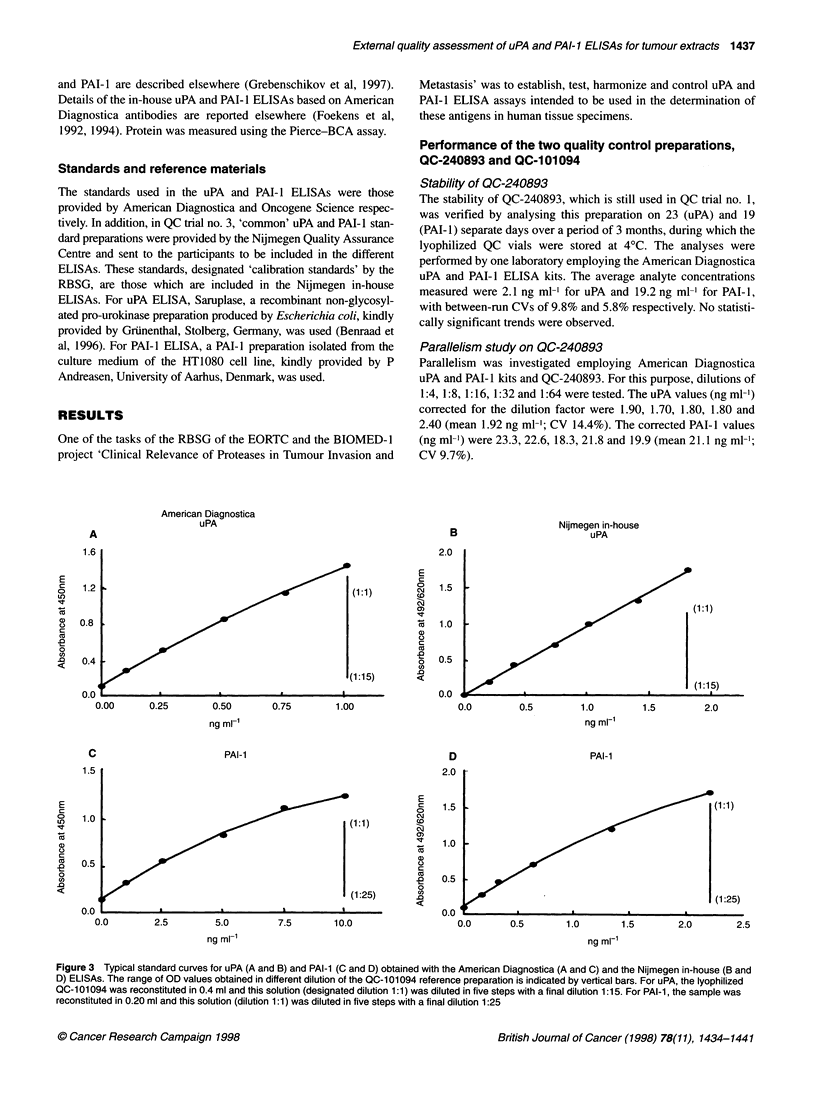
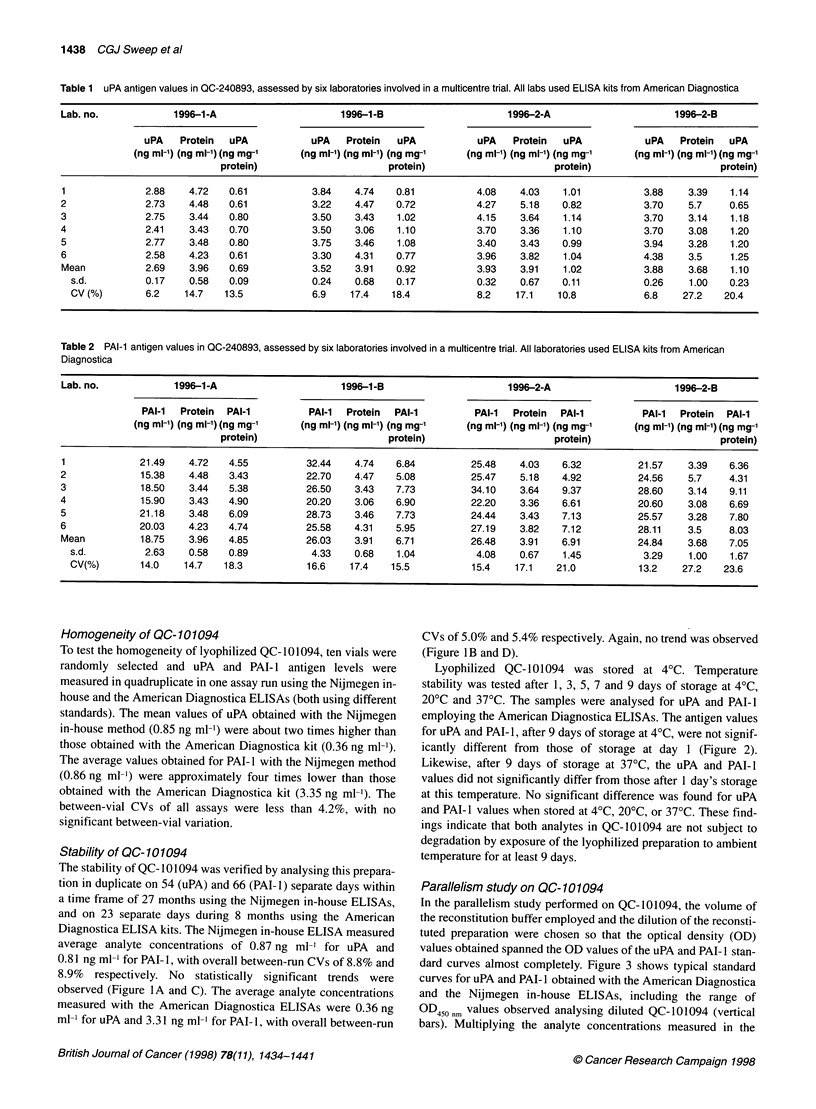
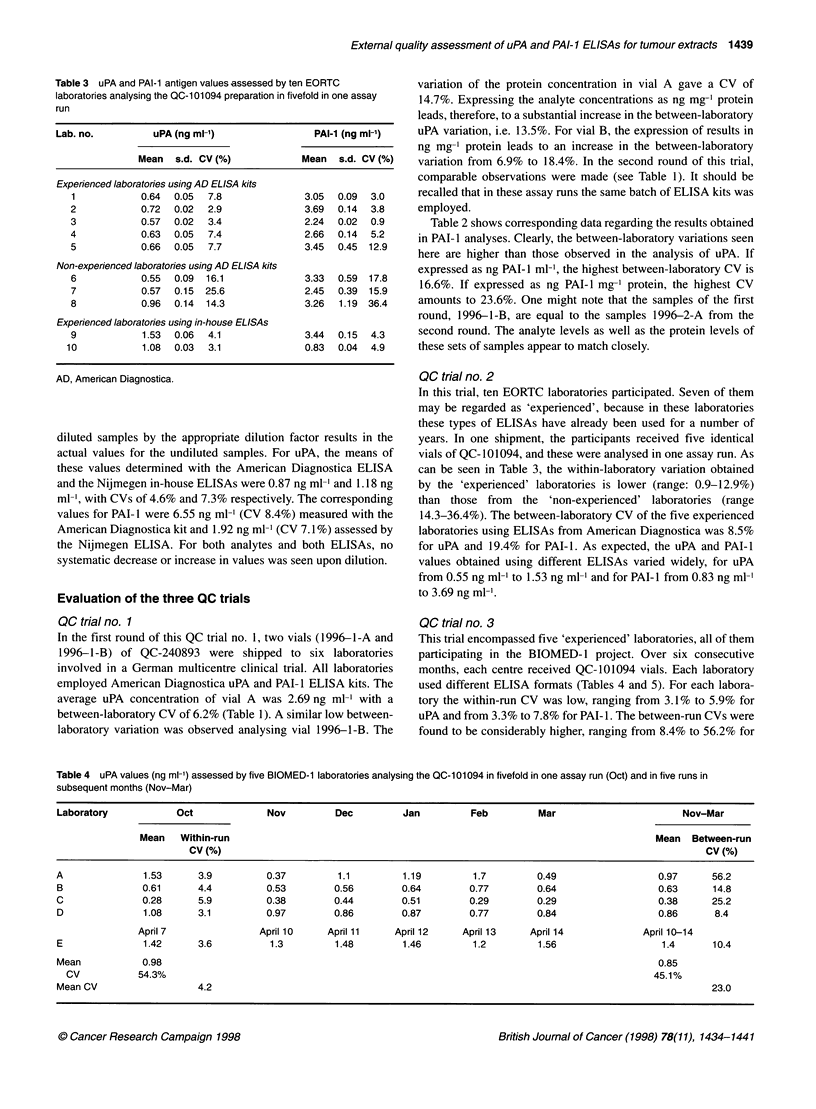
High levels of urokinase-type plasminogen activator (uPA) and plasminogen activator inhibitor type 1 (PAI-1) in breast cancer tissue extracts have been associated with rapid disease progression. In these studies, different enzyme-linked immunosorbent assay (ELISA) kits have been applied for the quantification, and consequently the ranges of uPA and PAI-1 levels reported differ considerably. Therefore, the Receptor and Biomarker Study Group (RBSG) of the European Organization for Research and Treatment of Cancer (EORTC) and a consortium of the BIOMED-1 project 'Clinical Relevance of Proteases in Tumor Invasion and Metastasis' initiated three collaborative between-laboratory assessment trials aimed at controlling uPA and PAI-1 antigen analyses. For this purpose, two control preparations were produced from different sources: pooled human breast cancer specimens (QC-240893) and human breast cancer xenografts raised in nude mice (QC-101094). The lyophilized preparations were stable for prolonged times (at least 3 and 27 months respectively) at 4 degrees C. Furthermore, a good parallelism following dilution was found for uPA and PAI-1. The data from QC trial no. 1 clearly indicated that acceptable between-laboratory coefficients of variation (CVs) for uPA (<8.2%) and PAI-1 (<16.6%) in QC-240893 could be achieved when the same type of ELISA kit (American Diagnostica) was used. From the second trial, in which ten EORTC laboratories each received five identical lyophilized QC-101094 samples, it appeared that the within-laboratory variations for uPA and PAI-1 determinations obtained by 'experienced' laboratories were lower (<12.9%) than those from non-experienced laboratories (<36.4%). In a third QC trial, five BIOMED-1 laboratories, all of which employed ELISA procedures for uPA and PAI-1, participated in six subsequent quality assessment rounds receiving five samples of QC-101094. Although for each laboratory the within-run CVs for uPA as well as for PAI-1 were low (<7.8%), the between-run CVs were found to be considerably higher (up to 56.2% for uPA and to 27.6% for PAI-1). Consequently, because of the different ELISA formats used, the absolute analyte values measured in the different laboratories varied substantially. The use of 'common external standards' in the different ELISAs resulted in a significant reduction of the between-laboratory CVs from 61.3% to 15.7% (uPA) and from 42.1% to 19.1% (PAI-1). The present data demonstrate that in multicentre studies the same ELISA kit should be used, and that external quality assurance (QA) is mandatory. Furthermore, it appears from the present study that standardization of the protein assay as a tissular parameter is imperative.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen P. A., Kjøller L., Christensen L., Duffy M. J. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer. 1997 Jul 3;72(1):1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Benraad T. J., Geurts-Moespot J., Grøndahl-Hansen J., Schmitt M., Heuvel J. J., de Witte J. H., Foekens J. A., Leake R. E., Brünner N., Sweep C. G. Immunoassays (ELISA) of urokinase-type plasminogen activator (uPA): report of an EORTC/BIOMED-1 workshop. Eur J Cancer. 1996 Jul;32A(8):1371–1381. doi: 10.1016/0959-8049(96)00118-9. [DOI] [PubMed] [Google Scholar]
- Bouchet C., Spyratos F., Martin P. M., Hacène K., Gentile A., Oglobine J. Prognostic value of urokinase-type plasminogen activator (uPA) and plasminogen activator inhibitors PAI-1 and PAI-2 in breast carcinomas. Br J Cancer. 1994 Feb;69(2):398–405. doi: 10.1038/bjc.1994.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy M. J., O'Grady P., Devaney D., O'Siorain L., Fennelly J. J., Lijnen H. J. Urokinase-plasminogen activator, a marker for aggressive breast carcinomas. Preliminary report. Cancer. 1988 Aug 1;62(3):531–533. doi: 10.1002/1097-0142(19880801)62:3<531::aid-cncr2820620315>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Duffy M. J. Proteases as prognostic markers in cancer. Clin Cancer Res. 1996 Apr;2(4):613–618. [PubMed] [Google Scholar]
- Foekens J. A., Look M. P., Peters H. A., van Putten W. L., Portengen H., Klijn J. G. Urokinase-type plasminogen activator and its inhibitor PAI-1: predictors of poor response to tamoxifen therapy in recurrent breast cancer. J Natl Cancer Inst. 1995 May 17;87(10):751–756. doi: 10.1093/jnci/87.10.751. [DOI] [PubMed] [Google Scholar]
- Foekens J. A., Schmitt M., van Putten W. L., Peters H. A., Bontenbal M., Jänicke F., Klijn J. G. Prognostic value of urokinase-type plasminogen activator in 671 primary breast cancer patients. Cancer Res. 1992 Nov 1;52(21):6101–6105. [PubMed] [Google Scholar]
- Foekens J. A., Schmitt M., van Putten W. L., Peters H. A., Kramer M. D., Jänicke F., Klijn J. G. Plasminogen activator inhibitor-1 and prognosis in primary breast cancer. J Clin Oncol. 1994 Aug;12(8):1648–1658. doi: 10.1200/JCO.1994.12.8.1648. [DOI] [PubMed] [Google Scholar]
- Grebenschikov N., Geurts-Moespot A., De Witte H., Heuvel J., Leake R., Sweep F., Benraad T. A sensitive and robust assay for urokinase and tissue-type plasminogen activators (uPA and tPA) and their inhibitor type I (PAI-1) in breast tumor cytosols. Int J Biol Markers. 1997 Jan-Mar;12(1):6–14. doi: 10.1177/172460089701200102. [DOI] [PubMed] [Google Scholar]
- Grøndahl-Hansen J., Christensen I. J., Rosenquist C., Brünner N., Mouridsen H. T., Danø K., Blichert-Toft M. High levels of urokinase-type plasminogen activator and its inhibitor PAI-1 in cytosolic extracts of breast carcinomas are associated with poor prognosis. Cancer Res. 1993 Jun 1;53(11):2513–2521. [PubMed] [Google Scholar]
- Jänicke F., Schmitt M., Graeff H. Clinical relevance of the urokinase-type and tissue-type plasminogen activators and of their type 1 inhibitor in breast cancer. Semin Thromb Hemost. 1991 Jul;17(3):303–312. doi: 10.1055/s-2007-1002624. [DOI] [PubMed] [Google Scholar]
- Jänicke F., Schmitt M., Pache L., Ulm K., Harbeck N., Höfler H., Graeff H. Urokinase (uPA) and its inhibitor PAI-1 are strong and independent prognostic factors in node-negative breast cancer. Breast Cancer Res Treat. 1993;24(3):195–208. doi: 10.1007/BF01833260. [DOI] [PubMed] [Google Scholar]
- Mignatti P., Rifkin D. B. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993 Jan;73(1):161–195. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- Romain S., Spyratos F., Lainé-Bidron C., Bouchet C., Guirou O., Martin P. M., Oglobine J., Magdelénat H. Comparative study of four extraction procedures for urokinase type plasminogen activator and plasminogen activator inhibitor-1 in breast cancer tissues. Eur J Clin Chem Clin Biochem. 1995 Sep;33(9):603–608. [PubMed] [Google Scholar]
- Schmitt M., Harbeck N., Thomssen C., Wilhelm O., Magdolen V., Reuning U., Ulm K., Höfler H., Jänicke F., Graeff H. Clinical impact of the plasminogen activation system in tumor invasion and metastasis: prognostic relevance and target for therapy. Thromb Haemost. 1997 Jul;78(1):285–296. [PubMed] [Google Scholar]
- Spyratos F., Martin P. M., Hacène K., Romain S., Andrieu C., Ferrero-Poüs M., Deytieux S., Le Doussal V., Tubiana-Hulin M., Brunet M. Multiparametric prognostic evaluation of biological factors in primary breast cancer. J Natl Cancer Inst. 1992 Aug 19;84(16):1266–1272. doi: 10.1093/jnci/84.16.1266. [DOI] [PubMed] [Google Scholar]


