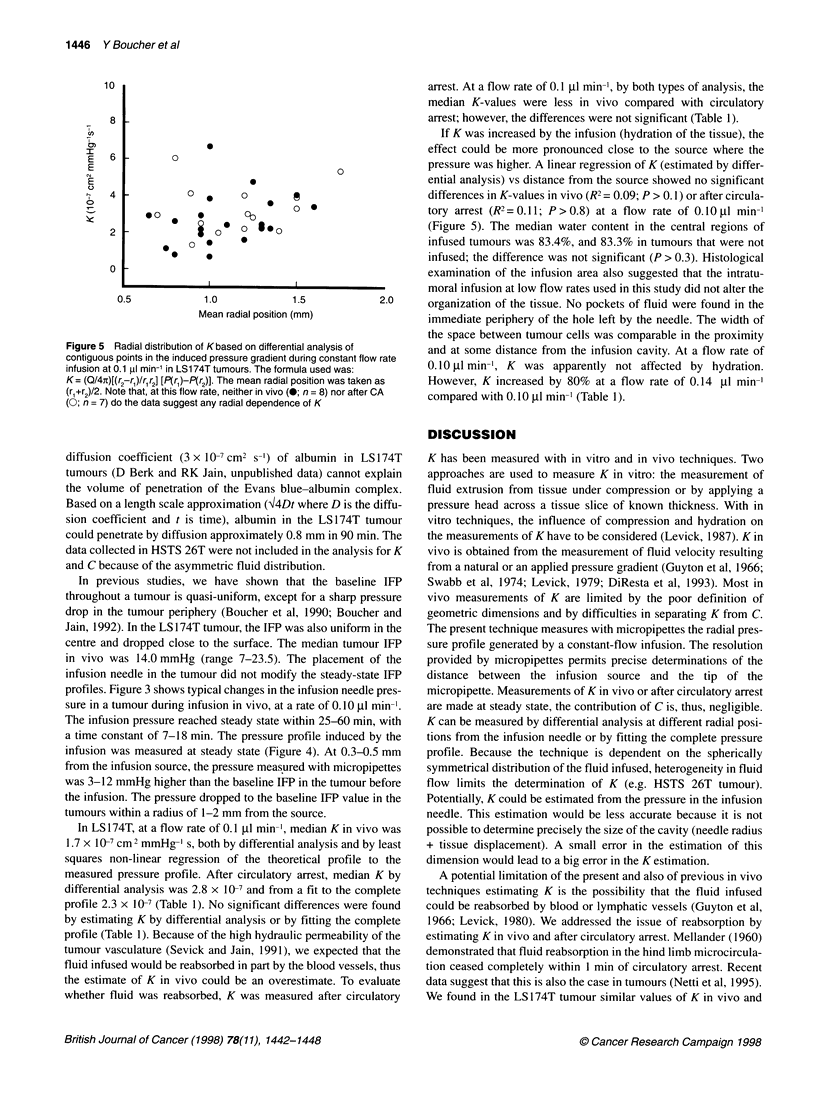
Abstract
We have developed a new technique to measure in vivo tumour tissue fluid transport parameters (hydraulic conductivity and compliance) that influence the systemic and intratumoral delivery of therapeutic agents. An infusion needle approximating a point source was constructed to produce a radially symmetrical fluid source in the centre of human tumours in immunodeficient mice. At constant flow, the pressure gradient generated in the tumour by the infusion of fluid (Evans blue-albumin in saline) was measured as a function of the radial position with micropipettes connected to a servo-null system. To evaluate whether the fluid infused was reabsorbed by blood vessels, infusions were also performed after circulatory arrest. In the colon adenocarcinoma LS174T with a spherically symmetrical distribution of Evans blue-albumin, the median hydraulic conductivity in vivo and after circulatory arrest at a flow rate of 0.1 microl min(-1) was, respectively, 1.7x10(-7) and 2.3x10(-7) cm2 mmHg(-1) s. Compliance estimates were 35 microl mmHg(-1) in vivo, and 100 microl mmHg(-1) after circulatory arrest. In the sarcoma HSTS 26T, hydraulic conductivity and compliance were not calculated because of the asymmetric distribution of the fluid infused. The technique will be helpful in identifying strategies to improve the intratumoral and systemic delivery of gene targeting vectors and other therapeutic agents.
Full text
PDF
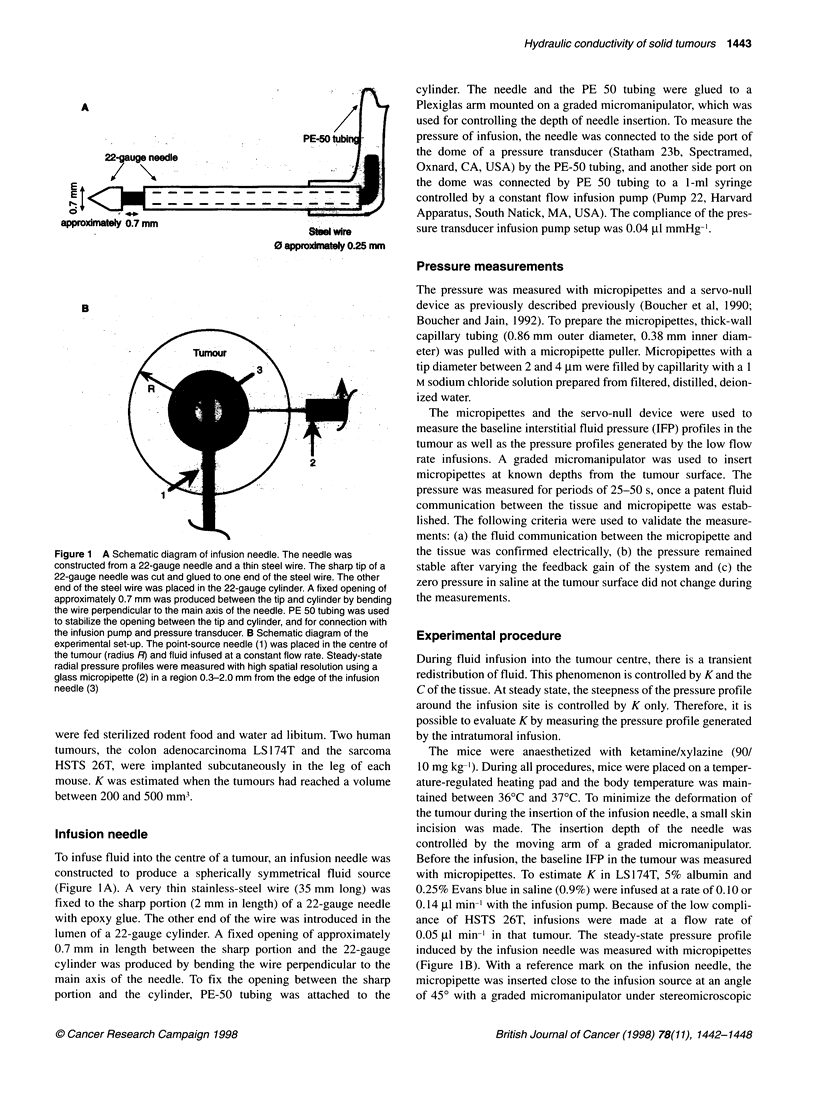
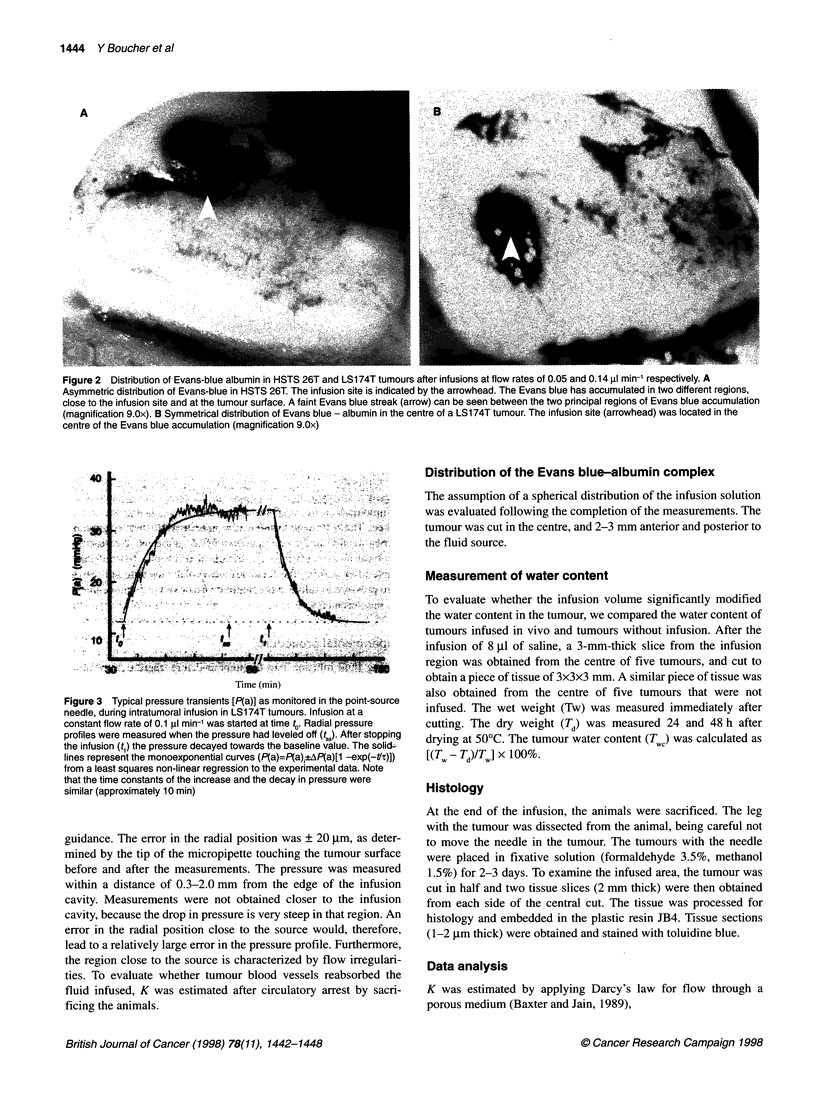
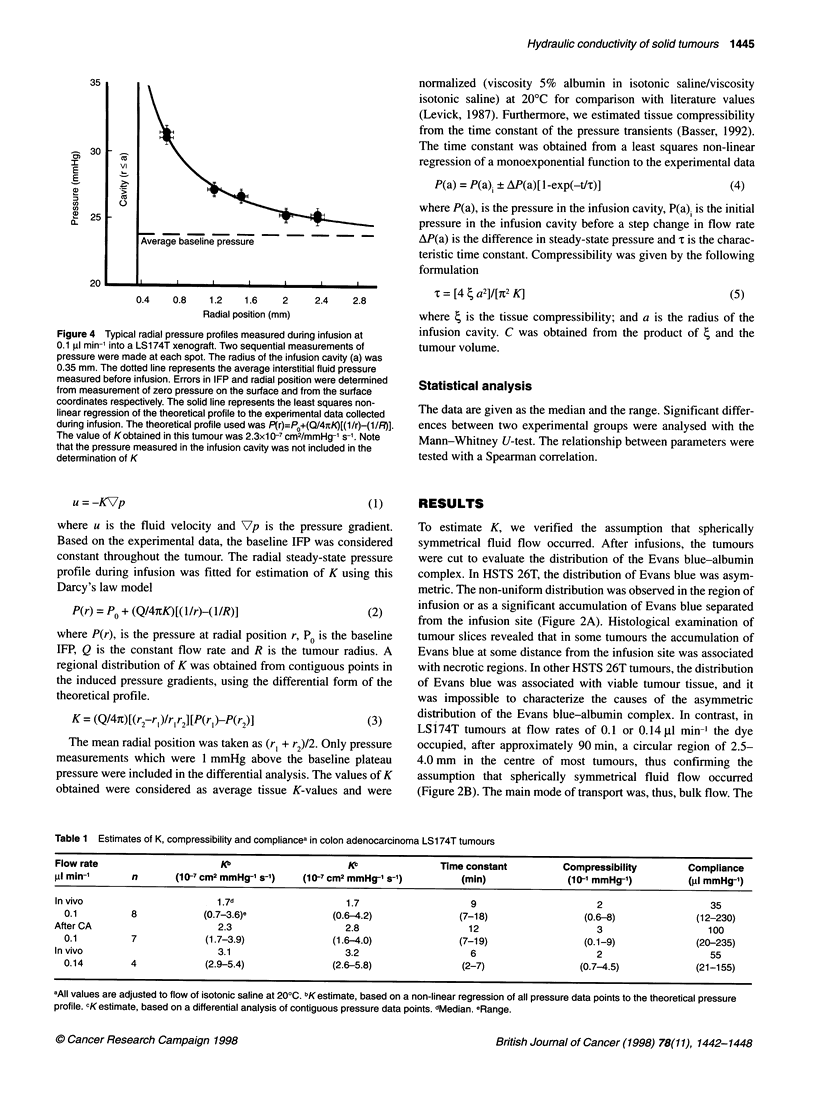


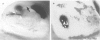
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basser P. J. Interstitial pressure, volume, and flow during infusion into brain tissue. Microvasc Res. 1992 Sep;44(2):143–165. doi: 10.1016/0026-2862(92)90077-3. [DOI] [PubMed] [Google Scholar]
- Baxter L. T., Jain R. K. Transport of fluid and macromolecules in tumors. I. Role of interstitial pressure and convection. Microvasc Res. 1989 Jan;37(1):77–104. doi: 10.1016/0026-2862(89)90074-5. [DOI] [PubMed] [Google Scholar]
- Bobo R. H., Laske D. W., Akbasak A., Morrison P. F., Dedrick R. L., Oldfield E. H. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher Y., Baxter L. T., Jain R. K. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res. 1990 Aug 1;50(15):4478–4484. [PubMed] [Google Scholar]
- Boucher Y., Jain R. K. Microvascular pressure is the principal driving force for interstitial hypertension in solid tumors: implications for vascular collapse. Cancer Res. 1992 Sep 15;52(18):5110–5114. [PubMed] [Google Scholar]
- DiResta G. R., Lee J., Larson S. M., Arbit E. Characterization of neuroblastoma xenograft in rat flank. I. Growth, interstitial fluid pressure, and interstitial fluid velocity distribution profiles. Microvasc Res. 1993 Sep;46(2):158–177. doi: 10.1006/mvre.1993.1044. [DOI] [PubMed] [Google Scholar]
- Fatt I. Dynamics of water transport in the corneal stroma. Exp Eye Res. 1968 Jul;7(3):402–412. doi: 10.1016/s0014-4835(68)80055-7. [DOI] [PubMed] [Google Scholar]
- Guyton A. C., Scheel K., Murphree D. Interstitial fluid pressure. 3. Its effect on resistance to tissue fluid mobility. Circ Res. 1966 Aug;19(2):412–419. doi: 10.1161/01.res.19.2.412. [DOI] [PubMed] [Google Scholar]
- Heise C., Sampson-Johannes A., Williams A., McCormick F., Von Hoff D. D., Kirn D. H. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997 Jun;3(6):639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- Jain R. K., Baxter L. T. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res. 1988 Dec 15;48(24 Pt 1):7022–7032. [PubMed] [Google Scholar]
- Jirtle R. L. Chemical modification of tumour blood flow. Int J Hyperthermia. 1988 Jul-Aug;4(4):355–371. doi: 10.3109/02656738809016490. [DOI] [PubMed] [Google Scholar]
- Lai-Fook S. J., Rochester N. L., Brown L. V. Effects of albumin, dextran, and hyaluronidase on pulmonary interstitial conductivity. J Appl Physiol (1985) 1989 Aug;67(2):606–613. doi: 10.1152/jappl.1989.67.2.606. [DOI] [PubMed] [Google Scholar]
- Levick J. R. Contributions of the lymphatic and microvascular systems to fluid absorption from the synovial cavity of the rabbit knee. J Physiol. 1980 Sep;306:445–461. doi: 10.1113/jphysiol.1980.sp013406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick J. R. Flow through interstitium and other fibrous matrices. Q J Exp Physiol. 1987 Oct;72(4):409–437. doi: 10.1113/expphysiol.1987.sp003085. [DOI] [PubMed] [Google Scholar]
- Levick J. R. The influence of hydrostatic pressure on trans-synovial fluid movement and on capsular expansion in the rabbit knee. J Physiol. 1979 Apr;289:69–82. doi: 10.1113/jphysiol.1979.sp012725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELLANDER S. Comparative studies on the adrenergic neuro-hormonal control of resistance and capacitance blood vessels in the cat. Acta Physiol Scand Suppl. 1960;50(176):1–86. [PubMed] [Google Scholar]
- Morrison P. F., Laske D. W., Bobo H., Oldfield E. H., Dedrick R. L. High-flow microinfusion: tissue penetration and pharmacodynamics. Am J Physiol. 1994 Jan;266(1 Pt 2):R292–R305. doi: 10.1152/ajpregu.1994.266.1.R292. [DOI] [PubMed] [Google Scholar]
- Netti P. A., Baxter L. T., Boucher Y., Skalak R., Jain R. K. Time-dependent behavior of interstitial fluid pressure in solid tumors: implications for drug delivery. Cancer Res. 1995 Nov 15;55(22):5451–5458. [PubMed] [Google Scholar]
- Nomura T., Nakajima S., Kawabata K., Yamashita F., Takakura Y., Hashida M. Intratumoral pharmacokinetics and in vivo gene expression of naked plasmid DNA and its cationic liposome complexes after direct gene transfer. Cancer Res. 1997 Jul 1;57(13):2681–2686. [PubMed] [Google Scholar]
- Pitt Ford T. R., Sachs J. R., Grotberg J. B., Glucksberg M. R. Perialveolar interstitial resistance and compliance in isolated rat lung. J Appl Physiol (1985) 1991 Jun;70(6):2750–2756. doi: 10.1152/jappl.1991.70.6.2750. [DOI] [PubMed] [Google Scholar]
- Sands H. Radiolabeled monoclonal antibodies for cancer therapy and diagnosis: is it really a chimera? J Nucl Med. 1992 Jan;33(1):29–32. [PubMed] [Google Scholar]
- Sevick E. M., Jain R. K. Measurement of capillary filtration coefficient in a solid tumor. Cancer Res. 1991 Feb 15;51(4):1352–1355. [PubMed] [Google Scholar]
- Swabb E. A., Wei J., Gullino P. M. Diffusion and convection in normal and neoplastic tissues. Cancer Res. 1974 Oct;34(10):2814–2822. [PubMed] [Google Scholar]
- Thompson W. D., Shiach K. J., Fraser R. A., McIntosh L. C., Simpson J. G. Tumours acquire their vasculature by vessel incorporation, not vessel ingrowth. J Pathol. 1987 Apr;151(4):323–332. doi: 10.1002/path.1711510413. [DOI] [PubMed] [Google Scholar]
- Tozer G. M., Lewis S., Michalowski A., Aber V. The relationship between regional variations in blood flow and histology in a transplanted rat fibrosarcoma. Br J Cancer. 1990 Feb;61(2):250–257. doi: 10.1038/bjc.1990.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola J. J., Agbaria R., Walbridge S., Oshiro E. M., Johns D. G., Kelley J. A., Oldfield E. H., Ram Z. In situ cyclopentenyl cytosine infusion for the treatment of experimental brain tumors. Cancer Res. 1995 Mar 15;55(6):1306–1309. [PubMed] [Google Scholar]
- Zawieja D. C., Garcia C., Granger H. J. Oxygen radicals, enzymes, and fluid transport through pericardial interstitium. Am J Physiol. 1992 Jan;262(1 Pt 2):H136–H143. doi: 10.1152/ajpheart.1992.262.1.H136. [DOI] [PubMed] [Google Scholar]