Abstract
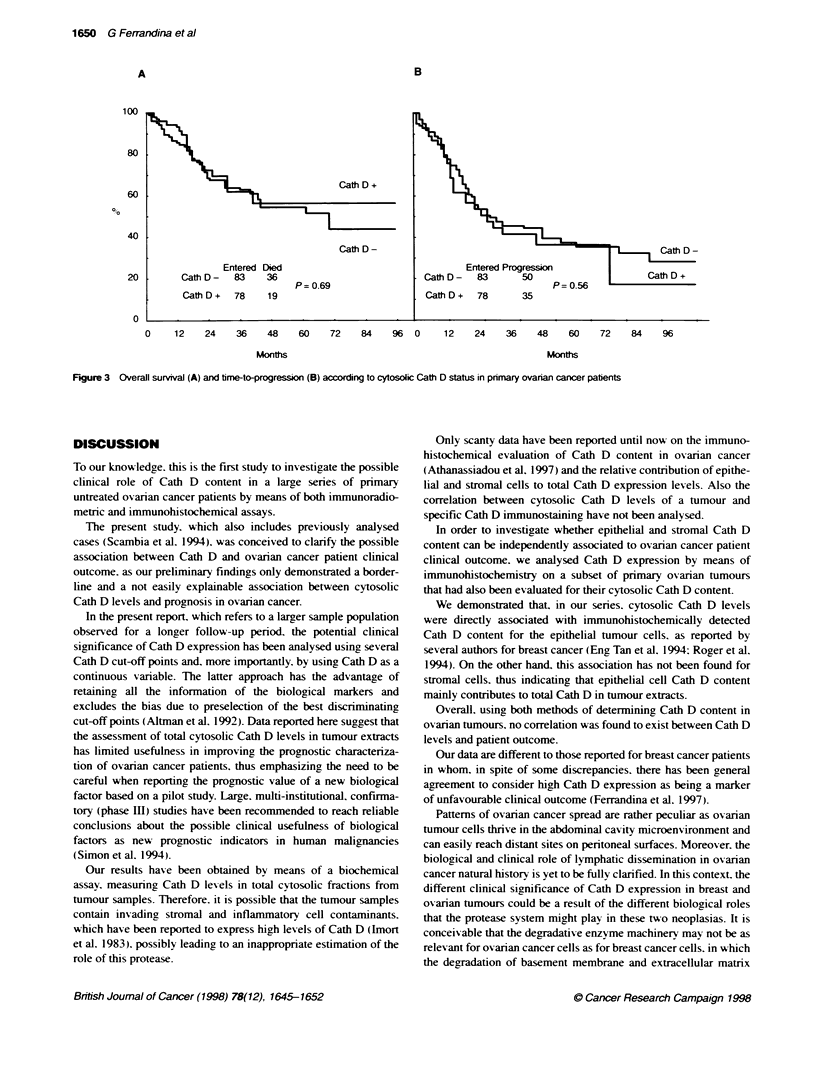
The aim of this study was to analyse the clinical significance of Cathepsin D (Cath D) content as determined by an immunoradiometric assay in a series of primary untreated ovarian cancers from 162 patients. In addition, immunohistochemical analysis of Cath D was also performed on a subset of 86 tumours. Cath D levels were distributed in an asymmetrical way and were skewed towards the lower values (median value 20.8 pmol mg(-1) protein, range 2.0-99.0 pmol mg(-1) protein). No correlation was found between Cath D levels and clinicopathological parameters. However, the percentage of Cath D positivity was significantly higher in oestrogen receptor-positive (57%) compared with oestrogen receptor-negative (36%) cases (P= 0.01). The percentage of Cath D-positive staining was not significantly different for both epithelial (27%) and stromal components (40%). Immunoradiometrically detected Cath D levels were not different according to Cath D stromal immunostaining (P= 0.18), while higher Cath D levels were measured in Cath D-positive than in Cath D-negative tumour epithelial cells (P = 0.027). Survival analysis was conducted on 161 primary untreated ovarian cancer patients. The 5-year overall survival rate was 57% and 55% in Cath D-positive and Cath D-negative patients respectively (P = 0.69). As far as time to progression was concerned, there was no significant difference in the survival rate of patients with either high or low Cath D content (P = 0.56). Similar results have been obtained in the subset of patients in which Cath D was analysed by immunohistochemistry. In conclusion, Cath D measurement in tumour extracts appears to have a limited usefulness in improving the prognostic characterization of ovarian cancer patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman D. G. Categorising continuous variables. Br J Cancer. 1991 Nov;64(5):975–975. doi: 10.1038/bjc.1991.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalbos D., Philips A., Galtier F., Rochefort H. Synthetic antiestrogens modulate induction of pS2 and cathepsin-D messenger ribonucleic acid by growth factors and adenosine 3',5'-monophosphate in MCF7 cells. Endocrinology. 1993 Aug;133(2):571–576. doi: 10.1210/endo.133.2.8344199. [DOI] [PubMed] [Google Scholar]
- Eng Tan P., Benz C. C., Dollbaum C., Moore D. H., 2nd, Edgerton S. M., Zava D. T., Thor A. D. Prognostic value of Cathepsin D expression in breast cancer: immunohistochemical assessment and correlation with radiometric assay. Ann Oncol. 1994 Apr;5(4):329–336. doi: 10.1093/oxfordjournals.annonc.a058836. [DOI] [PubMed] [Google Scholar]
- Ferrandina G., Scambia G., Bardelli F., Benedetti Panici P., Mancuso S., Messori A. Relationship between cathepsin-D content and disease-free survival in node-negative breast cancer patients: a meta-analysis. Br J Cancer. 1997;76(5):661–666. doi: 10.1038/bjc.1997.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtier-Dereure F., Capony F., Maudelonde T., Rochefort H. Estradiol stimulates cell growth and secretion of procathepsin D and a 120-kilodalton protein in the human ovarian cancer cell line BG-1. J Clin Endocrinol Metab. 1992 Dec;75(6):1497–1502. doi: 10.1210/jcem.75.6.1464654. [DOI] [PubMed] [Google Scholar]
- Garcia M., Derocq D., Pujol P., Rochefort H. Overexpression of transfected cathepsin D in transformed cells increases their malignant phenotype and metastatic potency. Oncogene. 1990 Dec;5(12):1809–1814. [PubMed] [Google Scholar]
- Gion M., Mione R., Dittadi R., Romanelli M., Pappagallo L., Capitanio G., Friede U., Barbazza R., Visonà A., Dante S. Relationship between cathepsin D and other pathological and biological parameters in 1752 patients with primary breast cancer. Eur J Cancer. 1995;31A(5):671–677. doi: 10.1016/0959-8049(94)00532-a. [DOI] [PubMed] [Google Scholar]
- Henzen-Logmans S. C., Fieret E. J., Berns E. M., van der Burg M. E., Klijn J. G., Foekens J. A. Ki-67 staining in benign, borderline, malignant primary and metastatic ovarian tumors: correlation with steroid receptors, epidermal-growth-factor receptor and cathepsin D. Int J Cancer. 1994 May 15;57(4):468–472. doi: 10.1002/ijc.2910570405. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Imort M., Zühlsdorf M., Feige U., Hasilik A., von Figura K. Biosynthesis and transport of lysosomal enzymes in human monocytes and macrophages. Effects of ammonium chloride, zymosan and tunicamycin. Biochem J. 1983 Sep 15;214(3):671–678. doi: 10.1042/bj2140671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isola J., Weitz S., Visakorpi T., Holli K., Shea R., Khabbaz N., Kallioniemi O. P. Cathepsin D expression detected by immunohistochemistry has independent prognostic value in axillary node-negative breast cancer. J Clin Oncol. 1993 Jan;11(1):36–43. doi: 10.1200/JCO.1993.11.1.36. [DOI] [PubMed] [Google Scholar]
- Johnson M. D., Westley B. R., May F. E. Oestrogenic activity of tamoxifen and its metabolites on gene regulation and cell proliferation in MCF-7 breast cancer cells. Br J Cancer. 1989 May;59(5):727–738. doi: 10.1038/bjc.1989.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn W., Pache L., Schmalfeldt B., Dettmar P., Schmitt M., Jänicke F., Graeff H. Urokinase (uPA) and PAI-1 predict survival in advanced ovarian cancer patients (FIGO III) after radical surgery and platinum-based chemotherapy. Gynecol Oncol. 1994 Dec;55(3 Pt 1):401–409. doi: 10.1006/gyno.1994.1313. [DOI] [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966 Mar;50(3):163–170. [PubMed] [Google Scholar]
- Maudelonde T., Escot C., Pujol P., Rouanet P., Defrenne A., Brouillet J. P., Rochefort H. In vivo stimulation by tamoxifen of cathepsin D RNA level in breast cancer. Eur J Cancer. 1994;30A(14):2049–2053. doi: 10.1016/0959-8049(94)00343-4. [DOI] [PubMed] [Google Scholar]
- Rochefort H., Capony F., Garcia M., Cavaillès V., Freiss G., Chambon M., Morisset M., Vignon F. Estrogen-induced lysosomal proteases secreted by breast cancer cells: a role in carcinogenesis? J Cell Biochem. 1987 Sep;35(1):17–29. doi: 10.1002/jcb.240350103. [DOI] [PubMed] [Google Scholar]
- Roger P., Montcourrier P., Maudelonde T., Brouillet J. P., Pages A., Laffargue F., Rochefort H. Cathepsin D immunostaining in paraffin-embedded breast cancer cells and macrophages: correlation with cytosolic assay. Hum Pathol. 1994 Sep;25(9):863–871. doi: 10.1016/0046-8177(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Scambia G., Benedetti-Panici P., Ferrandina G., Distefano M., Salerno G., Romanini M. E., Fagotti A., Mancuso S. Epidermal growth factor, oestrogen and progesterone receptor expression in primary ovarian cancer: correlation with clinical outcome and response to chemotherapy. Br J Cancer. 1995 Aug;72(2):361–366. doi: 10.1038/bjc.1995.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scambia G., Benedetti P., Ferrandina G., Battaglia F., Baiocchi G., Mancuso S. Cathepsin D assay in ovarian cancer: correlation with pathological features and receptors for oestrogen, progesterone and epidermal growth factor. Br J Cancer. 1991 Jul;64(1):182–184. doi: 10.1038/bjc.1991.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scambia G., Panici P. B., Ferrandina G., Salerno G., D'Agostino G., Distefano M., de Vincenzo R., Ercoli A., Mancuso S. Clinical significance of cathepsin D in primary ovarian cancer. Eur J Cancer. 1994;30A(7):935–940. doi: 10.1016/0959-8049(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Simon R., Altman D. G. Statistical aspects of prognostic factor studies in oncology. Br J Cancer. 1994 Jun;69(6):979–985. doi: 10.1038/bjc.1994.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyratos F., Maudelonde T., Brouillet J. P., Brunet M., Defrenne A., Andrieu C., Hacene K., Desplaces A., Rouëssé J., Rochefort H. Cathepsin D: an independent prognostic factor for metastasis of breast cancer. Lancet. 1989 Nov 11;2(8672):1115–1118. doi: 10.1016/s0140-6736(89)91487-6. [DOI] [PubMed] [Google Scholar]
- Vignon F., Capony F., Chambon M., Freiss G., Garcia M., Rochefort H. Autocrine growth stimulation of the MCF 7 breast cancer cells by the estrogen-regulated 52 K protein. Endocrinology. 1986 Apr;118(4):1537–1545. doi: 10.1210/endo-118-4-1537. [DOI] [PubMed] [Google Scholar]
- Westley B., Rochefort H. Estradiol induced proteins in the MCF7 human breast cancer cell line. Biochem Biophys Res Commun. 1979 Sep 27;90(2):410–416. doi: 10.1016/0006-291x(79)91250-6. [DOI] [PubMed] [Google Scholar]
- van der Stappen J. W., Williams A. C., Maciewicz R. A., Paraskeva C. Activation of cathepsin B, secreted by a colorectal cancer cell line requires low pH and is mediated by cathepsin D. Int J Cancer. 1996 Aug 7;67(4):547–554. doi: 10.1002/(SICI)1097-0215(19960807)67:4<547::AID-IJC14>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]