Abstract
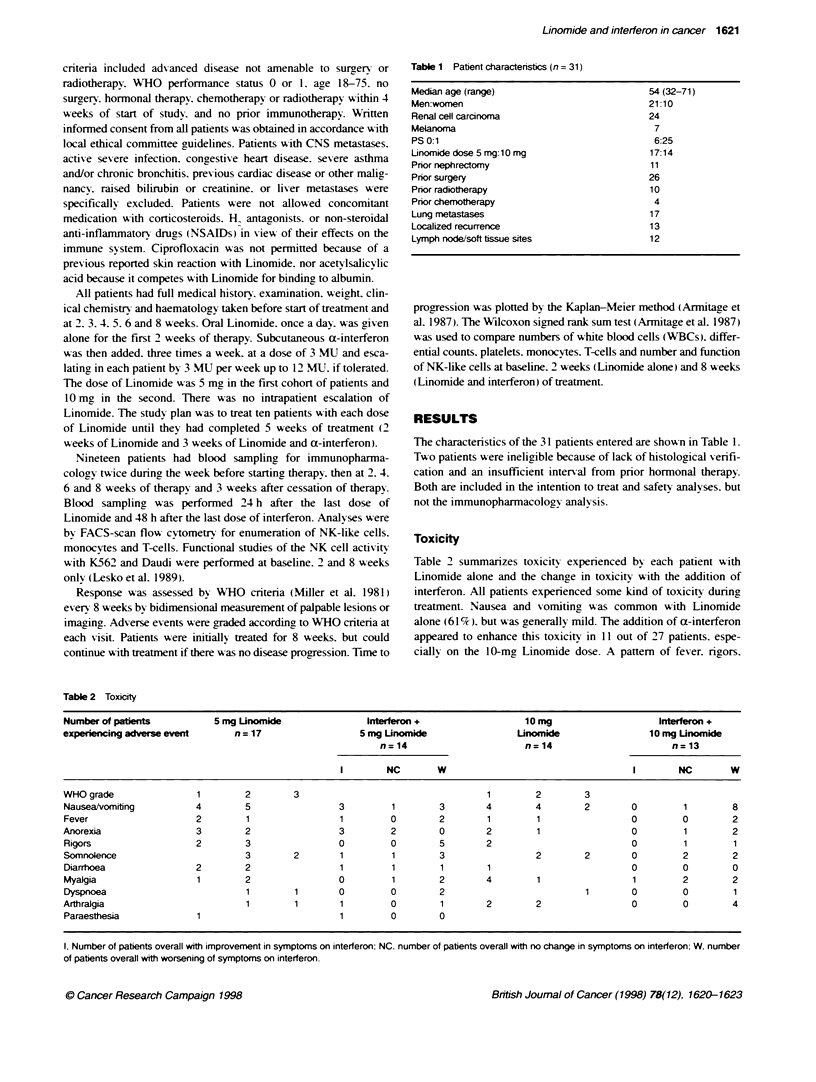
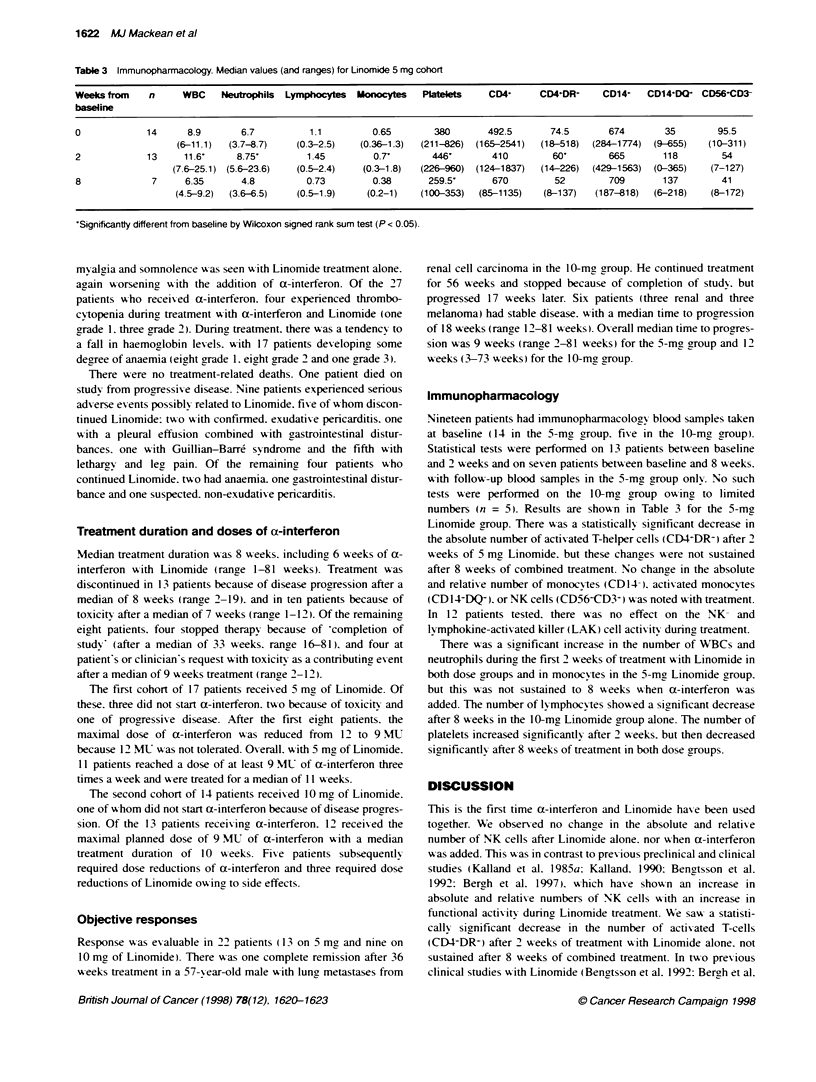
Thirty-one patients with advanced renal carcinoma or malignant melanoma were treated in the first feasibility study of alpha-interferon (Roferon) and the new oral immunomodulating agent, Linomide. Linomide 5 mg or 10 mg p.o. daily was given for 2 weeks; alpha-interferon was then added at 3 MU s.c. three times weekly, escalating in each patient by 3 MU per week, if tolerable, up to 12 MJ. The combination was poorly tolerated with nausea, vomiting, somnolence and myalgia commonly reported. Adverse events accounted for treatment withdrawal in ten patients and contributed to withdrawal in four other patients. Treatment with Linomide alone in the first 2 weeks led to a significant increase in white blood cells, neutrophils and platelets. When alpha-interferon was added, the platelet count decreased significantly over the following 6 weeks. Nineteen patients had white cell phenotype and function measured. After 2 weeks of 5 mg Linomide, a transient but significant decrease in the absolute number of activated T-helper cells (CD4+DR+) was observed. No changes in natural killer (NK) cell number or activity were observed. Twenty-two patients were evaluable for response. One with metastatic renal cell carcinoma had a complete response and six had stable disease. This study does not support the use of the combination because significant toxicity was seen without the anticipated immunological benefits.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bengtsson M., Simonsson B., Carlsson K., Nilsson B., Smedmyr B., Termander B., Oberg G., Tötterman T. H. Stimulation of NK cell, T cell, and monocyte functions by the novel immunomodulator Linomide after autologous bone marrow transplantation. A pilot study in patients with acute myeloid leukemia. Transplantation. 1992 Apr;53(4):882–888. doi: 10.1097/00007890-199204000-00032. [DOI] [PubMed] [Google Scholar]
- Bergh J. C., Tötterman T. H., Termander B. C., Strandgarden K. A., Gunnarsson P. O., Nilsson B. I. The first clinical pilot study of roquinimex (Linomide) in cancer patients with special focus on immunological effects. Cancer Invest. 1997;15(3):204–211. doi: 10.3109/07357909709039716. [DOI] [PubMed] [Google Scholar]
- Harning R., Koo G. C., Szalay J. Regulation of the metastasis of murine ocular melanoma by natural killer cells. Invest Ophthalmol Vis Sci. 1989 Sep;30(9):1909–1915. [PubMed] [Google Scholar]
- Harning R., Szalay J. A treatment for metastasis of murine ocular melanoma. Invest Ophthalmol Vis Sci. 1988 Oct;29(10):1505–1510. [PubMed] [Google Scholar]
- Horoszewicz J. S., Murphy G. P. An assessment of the current use of human interferons in therapy of urological cancers. J Urol. 1989 Nov;142(5):1173–1180. doi: 10.1016/s0022-5347(17)39022-5. [DOI] [PubMed] [Google Scholar]
- Kalland T., Alm G., Stålhandshe T. Augmentation of mouse natural killer cell activity by LS 2616, a new immunomodulator. J Immunol. 1985 Jun;134(6):3956–3961. [PubMed] [Google Scholar]
- Kalland T. Regulation of natural killer progenitors. Studies with a novel immunomodulator with distinct effects at the precursor level. J Immunol. 1990 Jun 1;144(11):4472–4476. [PubMed] [Google Scholar]
- Kirkwood J. M. Studies of interferons in the therapy of melanoma. Semin Oncol. 1991 Oct;18(5 Suppl 7):83–90. [PubMed] [Google Scholar]
- Larsson E. L., Joki A., Stålhandske T. Mechanism of action of the new immunomodulator LS2616 on T cell responses. Int J Immunopharmacol. 1987;9(4):425–431. doi: 10.1016/0192-0561(87)90016-6. [DOI] [PubMed] [Google Scholar]
- Lesko M. J., Lever R. S., Mackie R. M., Parrott D. M. The effect of topical steroid application on natural killer cell activity. Clin Exp Allergy. 1989 Nov;19(6):633–636. doi: 10.1111/j.1365-2222.1989.tb02759.x. [DOI] [PubMed] [Google Scholar]
- Miller A. B., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer. 1981 Jan 1;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Minasian L. M., Motzer R. J., Gluck L., Mazumdar M., Vlamis V., Krown S. E. Interferon alfa-2a in advanced renal cell carcinoma: treatment results and survival in 159 patients with long-term follow-up. J Clin Oncol. 1993 Jul;11(7):1368–1375. doi: 10.1200/JCO.1993.11.7.1368. [DOI] [PubMed] [Google Scholar]
- Oliver R. T. Renal cell cancer: is there long-term survival advantage from cytokine treatment? Eur J Cancer. 1994;30A(9):1214–1216. doi: 10.1016/0959-8049(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Whiteside T. L., Herberman R. B. Role of human natural killer cells in health and disease. Clin Diagn Lab Immunol. 1994 Mar;1(2):125–133. doi: 10.1128/cdli.1.2.125-133.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]



