Abstract
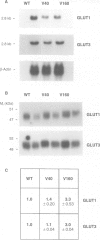
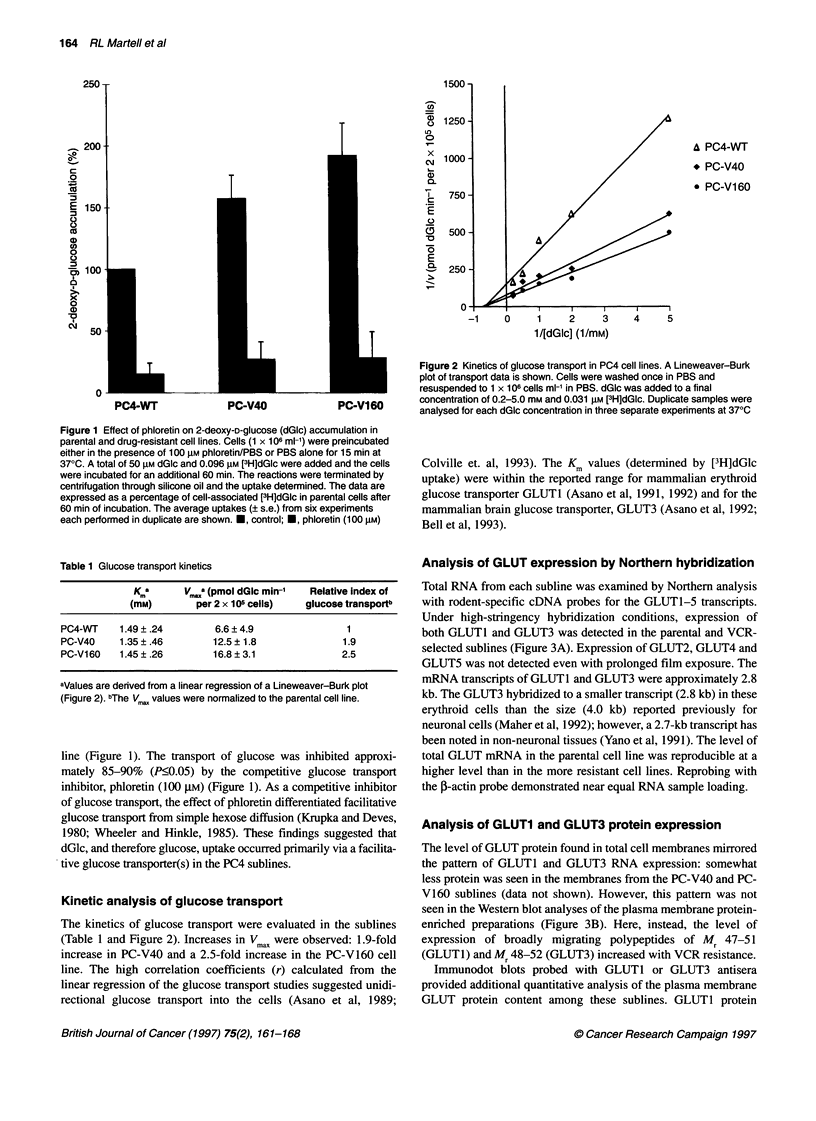
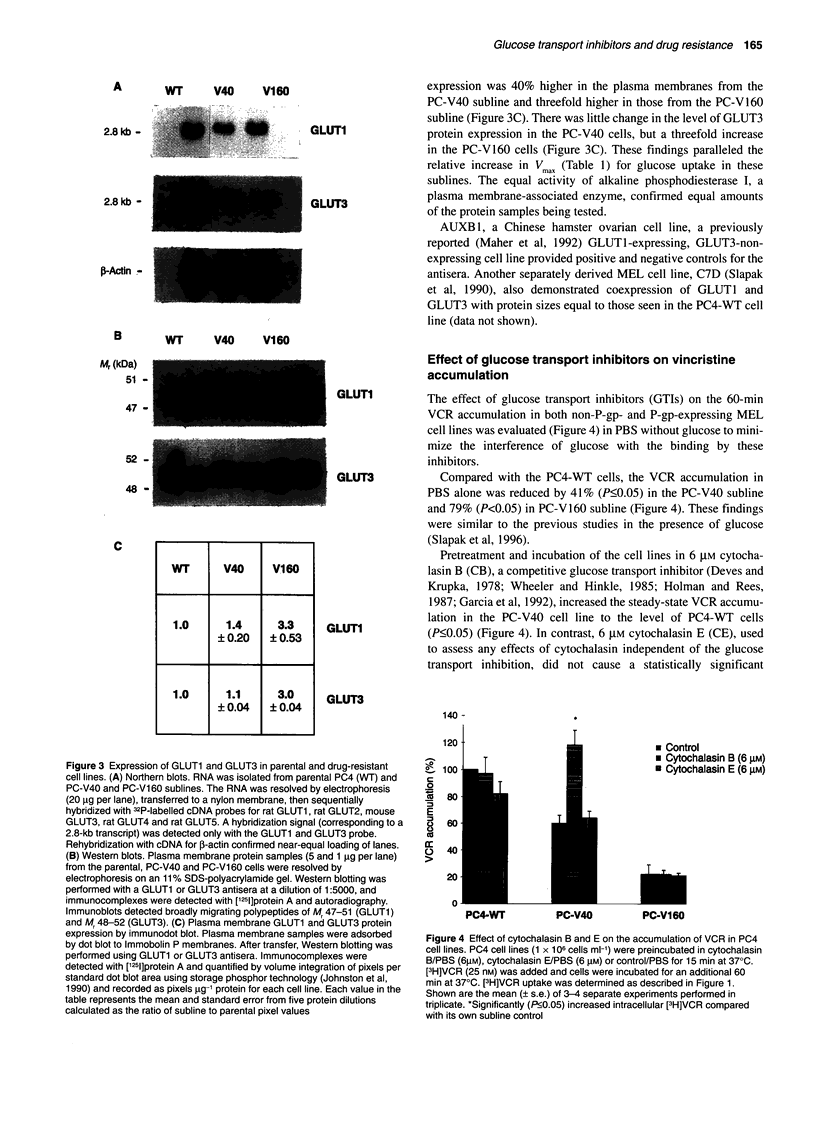
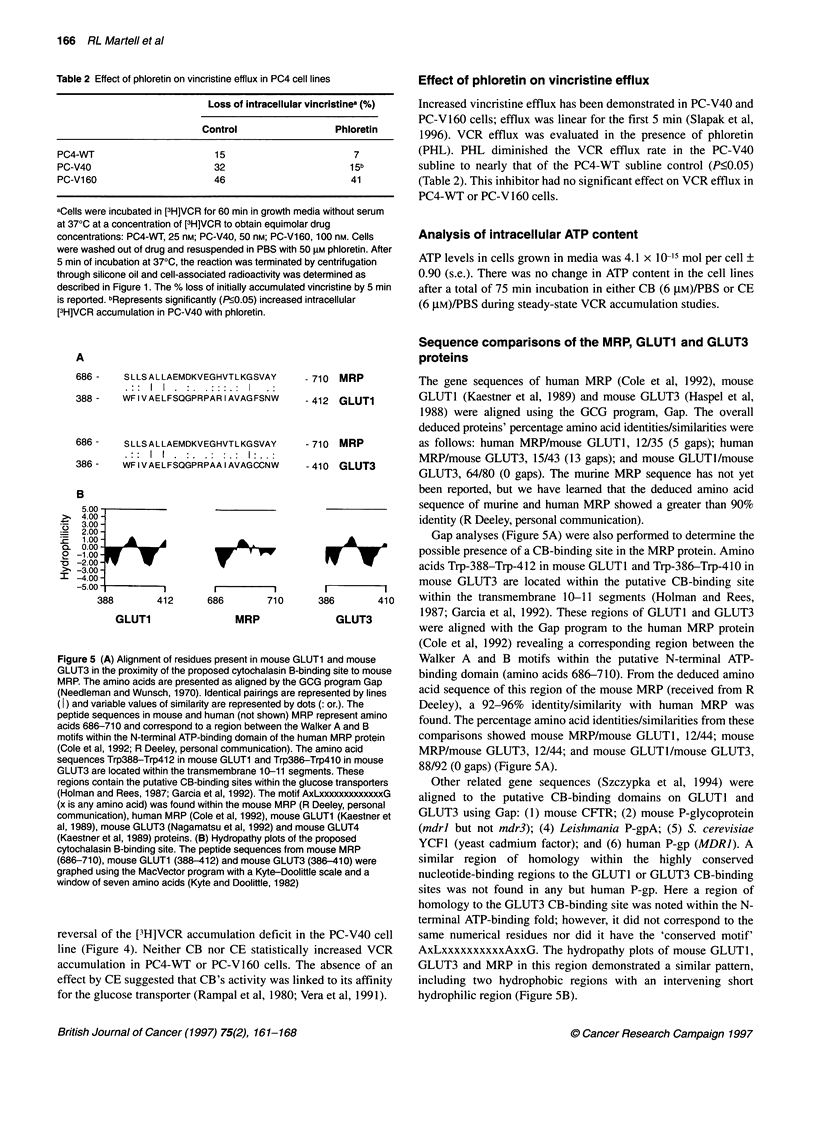
The relationship between mammalian facilitative glucose transport proteins (GLUT) and multidrug resistance was examined in two vincristine (VCR)-selected murine erythroleukaemia (MEL) PC4 cell lines. GLUT proteins, GLUT1 and GLUT3, were constitutively coexpressed in the parental cell line and also in the VCR-selected cell lines. Increased expression of the GLUT1 isoform was noted both in the PC-V40 (a non-P-glycoprotein, mrp-overexpressing subline) and in the more resistant PC-V160 (overexpressing mrp and mdr3) cell lines. Overexpression of GLUT3 was detected only in the PC-V160 subline. An increased rate of facilitative glucose transport (Vmax) and level of plasma membrane GLUT protein expression paralleled increased VCR resistance, active VCR efflux and decreased VCR steady-state accumulation in these cell lines. Glucose transport inhibitors (GTIs), cytochalasin B (CB) and phloretin blocked the active efflux and decreased steady-state accumulation of VCR in the PC-V40 subline. GTIs did not significantly affect VCR accumulation in the parental or PC-V160 cells. A comparison of protein sequences among GLUT1, GLUT3 and MRP revealed a putative cytochalasin B binding site in MRP, which displayed 44% sequence similarity/12% identity with that previously identified in GLUT1 and GLUT3; these regions also exhibited a similar hydropathy plot pattern. The findings suggested that CB bound to MRP and directly or indirectly lowered VCR efflux and/or CB bound to one or both GLUT proteins, which acted to lower the VCR efflux mediated by MRP. This is the first report of a non-neuronal murine cell line that expressed GLUT3.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano T., Katagiri H., Takata K., Tsukuda K., Lin J. L., Ishihara H., Inukai K., Hirano H., Yazaki Y., Oka Y. Characterization of GLUT3 protein expressed in Chinese hamster ovary cells. Biochem J. 1992 Nov 15;288(Pt 1):189–193. doi: 10.1042/bj2880189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T., Shibasaki Y., Lin J. L., Tsukuda K., Katagiri H., Ishihara H., Yazaki Y., Oka Y. Expression of the GLUT1 glucose transporter increases thymidine uptake in Chinese hamster ovary cells at low glucose concentrations. Cancer Res. 1991 Aug 15;51(16):4450–4454. [PubMed] [Google Scholar]
- Asano T., Shibasaki Y., Ohno S., Taira H., Lin J. L., Kasuga M., Kanazawa Y., Akanuma Y., Takaku F., Oka Y. Rabbit brain glucose transporter responds to insulin when expressed in insulin-sensitive Chinese hamster ovary cells. J Biol Chem. 1989 Feb 25;264(6):3416–3420. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992 Dec 4;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Sparks K. E., Fraser K., Loe D. W., Grant C. E., Wilson G. M., Deeley R. G. Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res. 1994 Nov 15;54(22):5902–5910. [PubMed] [Google Scholar]
- Colville C. A., Seatter M. J., Jess T. J., Gould G. W., Thomas H. M. Kinetic analysis of the liver-type (GLUT2) and brain-type (GLUT3) glucose transporters in Xenopus oocytes: substrate specificities and effects of transport inhibitors. Biochem J. 1993 Mar 15;290(Pt 3):701–706. doi: 10.1042/bj2900701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devés R., Krupka R. M. Cytochalasin B and the kinetics of inhibition of biological transport: a case of asymmetric binding to the glucose carrier. Biochim Biophys Acta. 1978 Jul 4;510(2):339–348. doi: 10.1016/0005-2736(78)90034-2. [DOI] [PubMed] [Google Scholar]
- Draye J. P., Courtoy P. J., Quintart J., Baudhuin P. Relations between plasma membrane and lysosomal membrane. 2. Quantitative evaluation of plasma membrane marker enzymes in the lysosomes. Eur J Biochem. 1987 Dec 30;170(1-2):405–411. doi: 10.1111/j.1432-1033.1987.tb13714.x. [DOI] [PubMed] [Google Scholar]
- Fanciulli M., Bruno T., Castiglione S., Del Carlo C., Paggi M. G., Floridi A. Glucose metabolism in adriamycin-sensitive and -resistant LoVo human colon carcinoma cells. Oncol Res. 1993;5(9):357–362. [PubMed] [Google Scholar]
- Garcia J. C., Strube M., Leingang K., Keller K., Mueckler M. M. Amino acid substitutions at tryptophan 388 and tryptophan 412 of the HepG2 (Glut1) glucose transporter inhibit transport activity and targeting to the plasma membrane in Xenopus oocytes. J Biol Chem. 1992 Apr 15;267(11):7770–7776. [PubMed] [Google Scholar]
- Gottesman M. M. How cancer cells evade chemotherapy: sixteenth Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1993 Feb 15;53(4):747–754. [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Brant A. M., Kahn B. B., Shepherd P. R., McCoid S. C., Gibbs E. M. Expression of the brain-type glucose transporter is restricted to brain and neuronal cells in mice. Diabetologia. 1992 Apr;35(4):304–309. doi: 10.1007/BF00401196. [DOI] [PubMed] [Google Scholar]
- Haspel H. C., Rosenfeld M. G., Rosen O. M. Characterization of antisera to a synthetic carboxyl-terminal peptide of the glucose transporter protein. J Biol Chem. 1988 Jan 5;263(1):398–403. [PubMed] [Google Scholar]
- Haspel H. C., Wilk E. W., Birnbaum M. J., Cushman S. W., Rosen O. M. Glucose deprivation and hexose transporter polypeptides of murine fibroblasts. J Biol Chem. 1986 May 25;261(15):6778–6789. [PubMed] [Google Scholar]
- Holman G. D., Rees W. D. Photolabelling of the hexose transporter at external and internal sites: fragmentation patterns and evidence for a conformational change. Biochim Biophys Acta. 1987 Mar 12;897(3):395–405. doi: 10.1016/0005-2736(87)90437-8. [DOI] [PubMed] [Google Scholar]
- Johnston R. F., Pickett S. C., Barker D. L. Autoradiography using storage phosphor technology. Electrophoresis. 1990 May;11(5):355–360. doi: 10.1002/elps.1150110503. [DOI] [PubMed] [Google Scholar]
- Juranka P. F., Zastawny R. L., Ling V. P-glycoprotein: multidrug-resistance and a superfamily of membrane-associated transport proteins. FASEB J. 1989 Dec;3(14):2583–2592. doi: 10.1096/fasebj.3.14.2574119. [DOI] [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., McLenithan J. C., Braiterman L. T., Cornelius P., Pekala P. H., Lane M. D. Sequence, tissue distribution, and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1989 May;86(9):3150–3154. doi: 10.1073/pnas.86.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn B. B., Flier J. S. Regulation of glucose-transporter gene expression in vitro and in vivo. Diabetes Care. 1990 Jun;13(6):548–564. doi: 10.2337/diacare.13.6.548. [DOI] [PubMed] [Google Scholar]
- Krupka R. M., Devés R. Asymmetric binding of steroids to internal and external sites in the glucose carrier of erythrocytes. Biochim Biophys Acta. 1980 May 8;598(1):134–144. doi: 10.1016/0005-2736(80)90271-0. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lyon R. C., Cohen J. S., Faustino P. J., Megnin F., Myers C. E. Glucose metabolism in drug-sensitive and drug-resistant human breast cancer cells monitored by magnetic resonance spectroscopy. Cancer Res. 1988 Feb 15;48(4):870–877. [PubMed] [Google Scholar]
- Maher F., Vannucci S., Takeda J., Simpson I. A. Expression of mouse-GLUT3 and human-GLUT3 glucose transporter proteins in brain. Biochem Biophys Res Commun. 1992 Jan 31;182(2):703–711. doi: 10.1016/0006-291x(92)91789-s. [DOI] [PubMed] [Google Scholar]
- Nagamatsu S., Kornhauser J. M., Burant C. F., Seino S., Mayo K. E., Bell G. I. Glucose transporter expression in brain. cDNA sequence of mouse GLUT3, the brain facilitative glucose transporter isoform, and identification of sites of expression by in situ hybridization. J Biol Chem. 1992 Jan 5;267(1):467–472. [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Pessin J. E., Bell G. I. Mammalian facilitative glucose transporter family: structure and molecular regulation. Annu Rev Physiol. 1992;54:911–930. doi: 10.1146/annurev.ph.54.030192.004403. [DOI] [PubMed] [Google Scholar]
- Rampal A. L., Pinkofsky H. B., Jung C. Y. Structure of cytochalasins and cytochalasin B binding sites in human erythrocyte membranes. Biochemistry. 1980 Feb 19;19(4):679–683. doi: 10.1021/bi00545a011. [DOI] [PubMed] [Google Scholar]
- Renner E. D., Plagemann P. G., Bernlohr R. W. Permeation of glucose by simple and facilitated diffusion by Novikoff rat hepatoma cells in suspension culture and its relationship to glucose metabolism. J Biol Chem. 1972 Sep 25;247(18):5765–5776. [PubMed] [Google Scholar]
- Schürmann A., Monden I., Joost H. G., Keller K. Subcellular distribution and activity of glucose transporter isoforms GLUT1 and GLUT4 transiently expressed in COS-7 cells. Biochim Biophys Acta. 1992 Jul 15;1131(3):245–252. doi: 10.1016/0167-4781(92)90022-r. [DOI] [PubMed] [Google Scholar]
- Slapak C. A., Daniel J. C., Levy S. B. Sequential emergence of distinct resistance phenotypes in murine erythroleukemia cells under adriamycin selection: decreased anthracycline uptake precedes increased P-glycoprotein expression. Cancer Res. 1990 Dec 15;50(24):7895–7901. [PubMed] [Google Scholar]
- Slapak C. A., Fracasso P. M., Martell R. L., Toppmeyer D. L., Lecerf J. M., Levy S. B. Overexpression of the multidrug resistance-associated protein (MRP) gene in vincristine but not doxorubicin-selected multidrug-resistant murine erythroleukemia cells. Cancer Res. 1994 Nov 1;54(21):5607–5613. [PubMed] [Google Scholar]
- Slapak C. A., Martell R. L., Terashima M., Levy S. B. Increased efflux of vincristine, but not of daunorubicin, associated with the murine multidrug resistance protein (MRP). Biochem Pharmacol. 1996 Nov 22;52(10):1569–1576. doi: 10.1016/s0006-2952(96)00561-8. [DOI] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Szczypka M. S., Wemmie J. A., Moye-Rowley W. S., Thiele D. J. A yeast metal resistance protein similar to human cystic fibrosis transmembrane conductance regulator (CFTR) and multidrug resistance-associated protein. J Biol Chem. 1994 Sep 9;269(36):22853–22857. [PubMed] [Google Scholar]
- Tew K. D. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994 Aug 15;54(16):4313–4320. [PubMed] [Google Scholar]
- Vera J. C., Castillo G. R., Rosen O. M. A possible role for a mammalian facilitative hexose transporter in the development of resistance to drugs. Mol Cell Biol. 1991 Jul;11(7):3407–3418. doi: 10.1128/mcb.11.7.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley A. R. The dynamics of the glucose transporter. Trends Biochem Sci. 1988 Jun;13(6):226–231. doi: 10.1016/0968-0004(88)90089-8. [DOI] [PubMed] [Google Scholar]
- Wheeler T. J., Hinkle P. C. The glucose transporter of mammalian cells. Annu Rev Physiol. 1985;47:503–517. doi: 10.1146/annurev.ph.47.030185.002443. [DOI] [PubMed] [Google Scholar]
- Yamada K., Tillotson L. G., Isselbacher K. J. Regulation of hexose carriers in chicken embryo fibroblasts. Effect of glucose starvation and role of protein synthesis. J Biol Chem. 1983 Aug 25;258(16):9786–9792. [PubMed] [Google Scholar]
- Yano H., Seino Y., Inagaki N., Hinokio Y., Yamamoto T., Yasuda K., Masuda K., Someya Y., Imura H. Tissue distribution and species difference of the brain type glucose transporter (GLUT3). Biochem Biophys Res Commun. 1991 Jan 31;174(2):470–477. doi: 10.1016/0006-291x(91)91440-n. [DOI] [PubMed] [Google Scholar]