Abstract
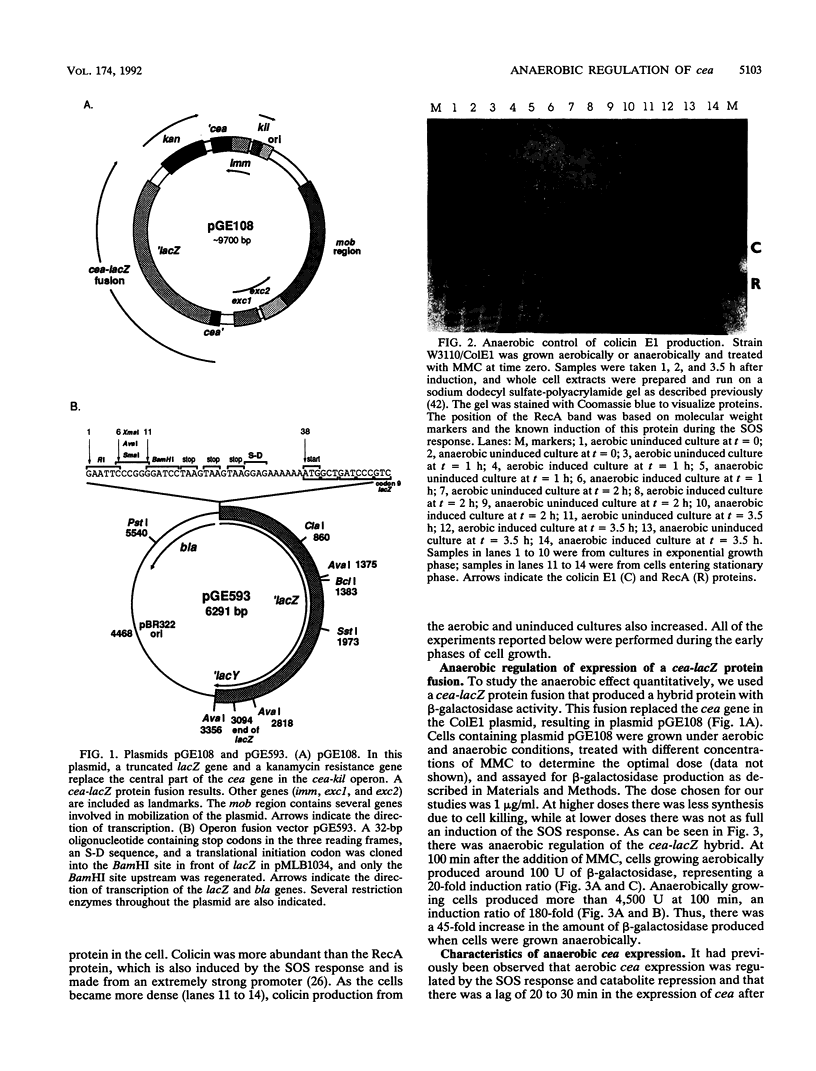
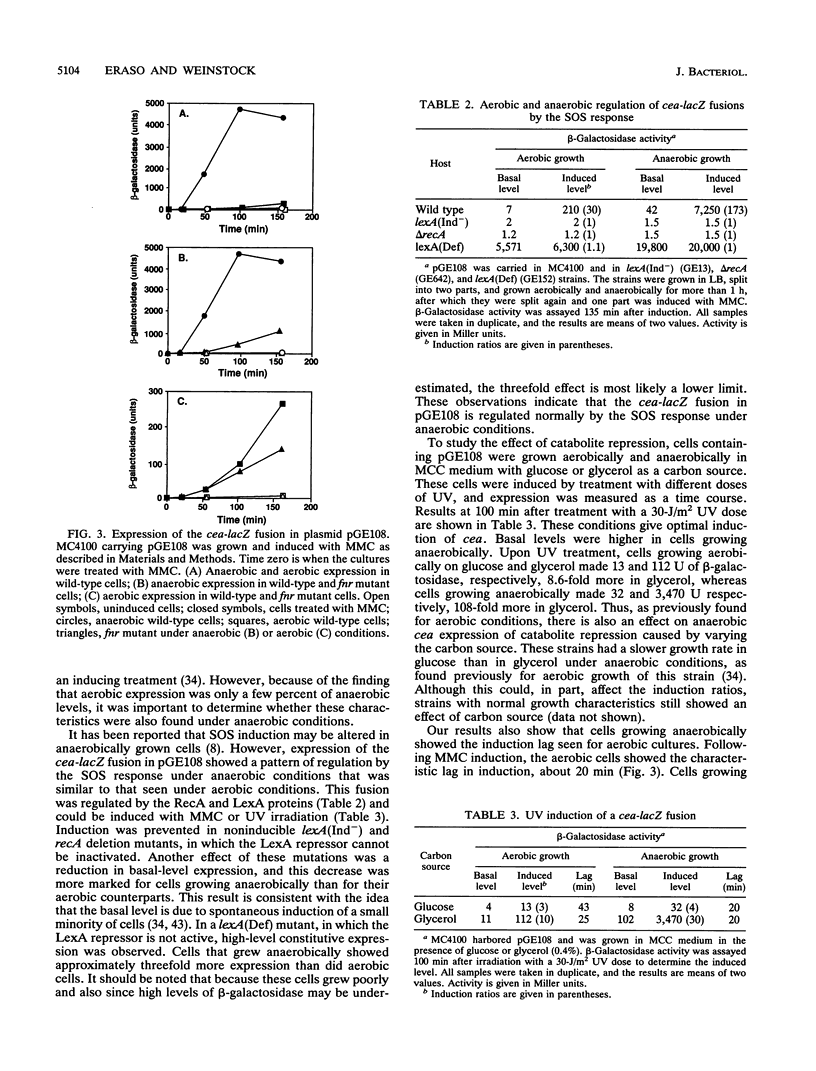
Expression of the cea gene, which is carried by the ColE1 plasmid and which encodes colicin E1, was found to be greatly increased when the cells were grown anaerobically. By using cea-lacZ fusions to quantitate expression, aerobic levels were found to be only a few percent of the anaerobic levels. The anaerobic increase in expression was observed both in protein and in operon fusions, indicating that its regulation occurred at the level of transcription. It was also found to require a functional fnr gene and to occur when the cea-lacZ fusion was present as a single copy in the bacterial chromosome instead of in the multicopy ColE1 plasmid. Anaerobic expression was regulated by the SOS response and catabolite repression as is aerobic expression. The start site of the mRNA produced under anaerobic conditions was mapped by primer extension and found to be the same as the start for mRNA produced under aerobic conditions. These observations show that the cea gene is anaerobically regulated and that the Fnr protein is a positive regulator of transcription of this gene.
Full text
PDF

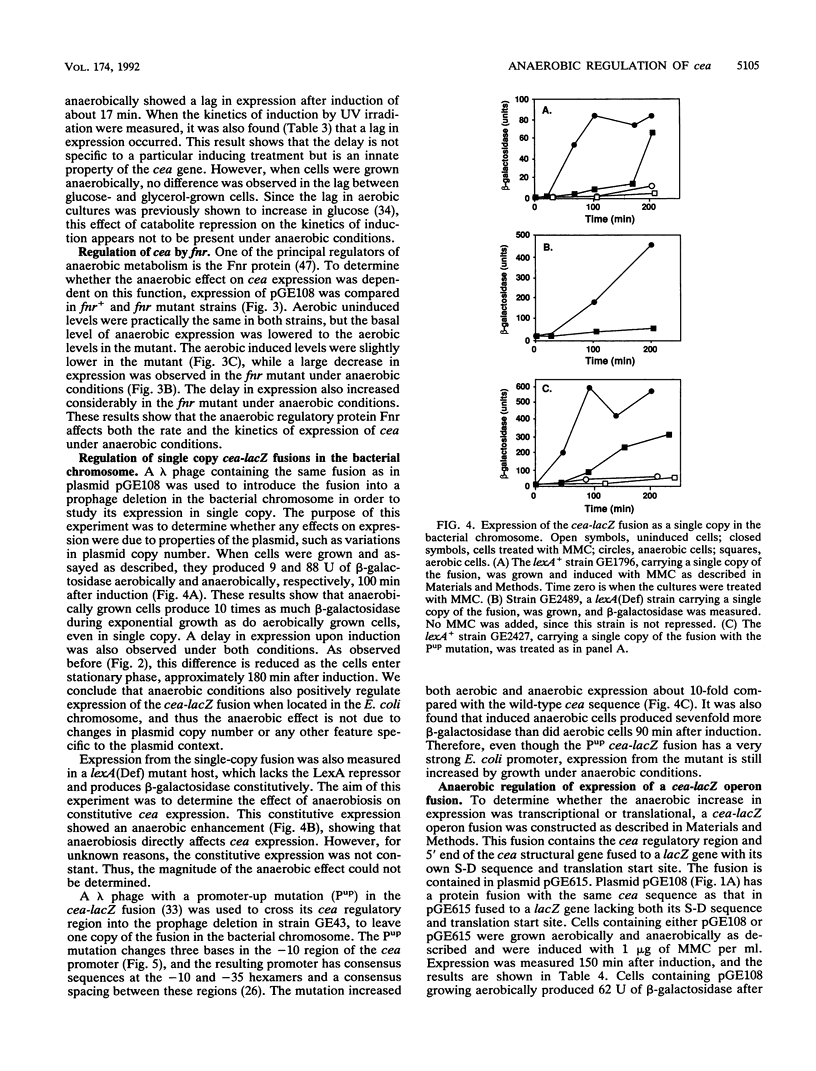
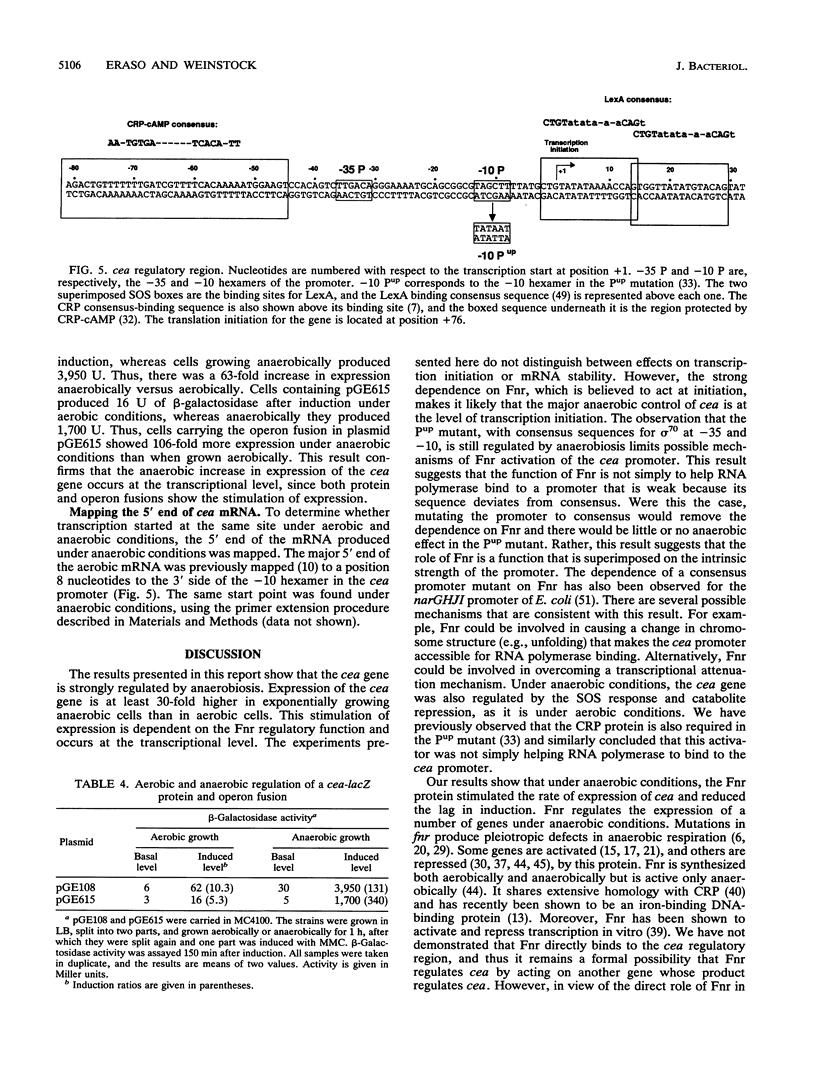



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J., Kinney T., Thompson S., Rubin L., Helling R. B. Frequency-Dependent Selection for Plasmid-Containing Cells of ESCHERICHIA COLI. Genetics. 1979 Apr;91(4):627–637. doi: 10.1093/genetics/91.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg B. L., Li J., Heider J., Stewart V. Nitrate-inducible formate dehydrogenase in Escherichia coli K-12. I. Nucleotide sequence of the fdnGHI operon and evidence that opal (UGA) encodes selenocysteine. J Biol Chem. 1991 Nov 25;266(33):22380–22385. [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Chan P. T., Ohmori H., Tomizawa J., Lebowitz J. Nucleotide sequence and gene organization of ColE1 DNA. J Biol Chem. 1985 Jul 25;260(15):8925–8935. [PubMed] [Google Scholar]
- Chippaux M., Giudici D., Abou-Jaoudé A., Casse F., Pascal M. C. Laboratoire de Chimie Bactérienne C.N.R.S., Marsielle, France. Mol Gen Genet. 1978 Apr 6;160(2):225–229. doi: 10.1007/BF00267485. [DOI] [PubMed] [Google Scholar]
- Droffner M. L., Yamamoto N. Anaerobic cultures of Salmonella typhimurium do not exhibit inducible proteolytic function of the recA gene and recBC function. J Bacteriol. 1983 Nov;156(2):962–965. doi: 10.1128/jb.156.2.962-965.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Kishi F., Nakazawa A. Direct participation of lexA protein in repression of colicin E1 synthesis. J Bacteriol. 1982 Jun;150(3):1479–1481. doi: 10.1128/jb.150.3.1479-1481.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Nakazawa A. Cyclic AMP-dependent initiation and rho-dependent termination of colicin E1 gene transcription. J Biol Chem. 1983 Jun 10;258(11):7072–7078. [PubMed] [Google Scholar]
- Ebina Y., Takahara Y., Kishi F., Nakazawa A., Brent R. LexA protein is a repressor of the colicin E1 gene. J Biol Chem. 1983 Nov 10;258(21):13258–13261. [PubMed] [Google Scholar]
- Eiglmeier K., Honoré N., Iuchi S., Lin E. C., Cole S. T. Molecular genetic analysis of FNR-dependent promoters. Mol Microbiol. 1989 Jul;3(7):869–878. doi: 10.1111/j.1365-2958.1989.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Green J., Trageser M., Six S., Unden G., Guest J. R. Characterization of the FNR protein of Escherichia coli, an iron-binding transcriptional regulator. Proc Biol Sci. 1991 May 22;244(1310):137–144. doi: 10.1098/rspb.1991.0062. [DOI] [PubMed] [Google Scholar]
- Hull R. A. Effect of tsl mutations on Col E1 expression in a recA strain of Escherichia coli K-12. J Bacteriol. 1975 Aug;123(2):775–776. doi: 10.1128/jb.123.2.775-776.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman P. S., Peakman T. C., Busby S. J., Quincey R. V., Cole J. A. Location and sequence of the promoter of the gene for the NADH-dependent nitrite reductase of Escherichia coli and its regulation by oxygen, the Fnr protein and nitrite. J Mol Biol. 1987 Aug 20;196(4):781–788. doi: 10.1016/0022-2836(87)90404-9. [DOI] [PubMed] [Google Scholar]
- Jennings M. P., Beacham I. R. Analysis of the Escherichia coli gene encoding L-asparaginase II, ansB, and its regulation by cyclic AMP receptor and FNR proteins. J Bacteriol. 1990 Mar;172(3):1491–1498. doi: 10.1128/jb.172.3.1491-1498.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. M., Gunsalus R. P. Regulation of Escherichia coli fumarate reductase (frdABCD) operon expression by respiratory electron acceptors and the fnr gene product. J Bacteriol. 1987 Jul;169(7):3340–3349. doi: 10.1128/jb.169.7.3340-3349.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Krueger J. H., Elledge S. J., Walker G. C. Isolation and characterization of Tn5 insertion mutations in the lexA gene of Escherichia coli. J Bacteriol. 1983 Mar;153(3):1368–1378. doi: 10.1128/jb.153.3.1368-1378.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Guest J. R. Mutants of Escherichia coli K12 unable to use fumarate as an anaerobic electron acceptor. J Gen Microbiol. 1976 Dec;97(2):145–160. doi: 10.1099/00221287-97-2-145. [DOI] [PubMed] [Google Scholar]
- Li S. F., DeMoss J. A. Promoter region of the nar operon of Escherichia coli: nucleotide sequence and transcription initiation signals. J Bacteriol. 1987 Oct;169(10):4614–4620. doi: 10.1128/jb.169.10.4614-4620.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Lotz W. Effect of guanosine tetraphosphate on in vitro protein synthesis directed by E1 and E3 colicinogenic factors. J Bacteriol. 1978 Aug;135(2):707–712. doi: 10.1128/jb.135.2.707-712.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. E., Hawley D. K., Entriken R., McClure W. R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa A., Suzuki N., Tamada T. Requirements of glucose and incubation under static conditions for optimal colicin E1 induction. Antimicrob Agents Chemother. 1977 Feb;11(2):219–224. doi: 10.1128/aac.11.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa A., Tamada T. Stimulation of colicin E 1 synthesis by cyclic 3', 5'-adenosine monophosphate in mitomycin C-induced Escherichia coli. Biochem Biophys Res Commun. 1972 Jan 31;46(2):1004–1010. doi: 10.1016/s0006-291x(72)80241-9. [DOI] [PubMed] [Google Scholar]
- Newman B. M., Cole J. A. The chromosomal location and pleiotropic effects of mutations of the nirA+ gene of Escherichia coli K12: the essential role of nirA+ in nitrite reduction and in other anaerobic redox reactions. J Gen Microbiol. 1978 May;106(1):1–12. doi: 10.1099/00221287-106-1-1. [DOI] [PubMed] [Google Scholar]
- Sabik J. F., Suit J. L., Luria S. E. cea-kil operon of the ColE1 plasmid. J Bacteriol. 1983 Mar;153(3):1479–1485. doi: 10.1128/jb.153.3.1479-1485.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles B., Weinstock G. M. Interaction of the CRP-cAMP complex with the cea regulatory region. Mol Gen Genet. 1989 Feb;215(3):537–542. doi: 10.1007/BF00427053. [DOI] [PubMed] [Google Scholar]
- Salles B., Weinstock G. M. Mutation of the promoter and LexA binding sites of cea, the gene encoding colicin E1. Mol Gen Genet. 1989 Feb;215(3):483–489. doi: 10.1007/BF00427047. [DOI] [PubMed] [Google Scholar]
- Salles B., Weisemann J. M., Weinstock G. M. Temporal control of colicin E1 induction. J Bacteriol. 1987 Nov;169(11):5028–5034. doi: 10.1128/jb.169.11.5028-5034.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers G., Böck A. Novel transcriptional control of the pyruvate formate-lyase gene: upstream regulatory sequences and multiple promoters regulate anaerobic expression. J Bacteriol. 1989 May;171(5):2485–2498. doi: 10.1128/jb.171.5.2485-2498.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers R. G., Zehelein E., Böck A. Two-dimensional gel electrophoretic analysis of Escherichia coli proteins: influence of various anaerobic growth conditions and the fnr gene product on cellular protein composition. Arch Microbiol. 1988 Jan;149(3):240–244. doi: 10.1007/BF00422011. [DOI] [PubMed] [Google Scholar]
- Shafferman A., Flashner Y., Cohen S. ColE1 DNA sequences interacting in cis, essential for mitomycin-C induced lethality. Mol Gen Genet. 1979 Oct 2;176(1):139–146. doi: 10.1007/BF00334305. [DOI] [PubMed] [Google Scholar]
- Sharrocks A. D., Green J., Guest J. R. FNR activates and represses transcription in vitro. Proc Biol Sci. 1991 Sep 23;245(1314):219–226. doi: 10.1098/rspb.1991.0113. [DOI] [PubMed] [Google Scholar]
- Shaw D. J., Rice D. W., Guest J. R. Homology between CAP and Fnr, a regulator of anaerobic respiration in Escherichia coli. J Mol Biol. 1983 May 15;166(2):241–247. doi: 10.1016/s0022-2836(83)80011-4. [DOI] [PubMed] [Google Scholar]
- Shirabe K., Ebina Y., Miki T., Nakazawa T., Nakazawa A. Positive regulation of the colicin E1 gene by cyclic AMP and cyclic AMP receptor protein. Nucleic Acids Res. 1985 Jul 11;13(13):4687–4698. doi: 10.1093/nar/13.13.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R., Zhang S. P., Krueger J., Zubay G. Colicin synthesis and cell death. J Bacteriol. 1985 Jul;163(1):167–173. doi: 10.1128/jb.163.1.167-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. Regulation and over-expression of the fnr gene of Escherichia coli. J Gen Microbiol. 1987 Dec;133(12):3279–3288. doi: 10.1099/00221287-133-12-3279. [DOI] [PubMed] [Google Scholar]
- Spiro S., Roberts R. E., Guest J. R. FNR-dependent repression of the ndh gene of Escherichia coli and metal ion requirement for FNR-regulated gene expression. Mol Microbiol. 1989 May;3(5):601–608. doi: 10.1111/j.1365-2958.1989.tb00207.x. [DOI] [PubMed] [Google Scholar]
- Stewart V. Nitrate respiration in relation to facultative metabolism in enterobacteria. Microbiol Rev. 1988 Jun;52(2):190–232. doi: 10.1128/mr.52.2.190-232.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V. Requirement of Fnr and NarL functions for nitrate reductase expression in Escherichia coli K-12. J Bacteriol. 1982 Sep;151(3):1320–1325. doi: 10.1128/jb.151.3.1320-1325.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suit J. L., Fan M. L., Sabik J. F., Labarre R., Luria S. E. Alternative forms of lethality in mitomycin C-induced bacteria carrying ColE1 plasmids. Proc Natl Acad Sci U S A. 1983 Jan;80(2):579–583. doi: 10.1073/pnas.80.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. S., DeMoss J. A. Promoter sequence requirements for Fnr-dependent activation of transcription of the narGHJI operon. Mol Microbiol. 1991 Feb;5(2):353–360. doi: 10.1111/j.1365-2958.1991.tb02116.x. [DOI] [PubMed] [Google Scholar]
- Walker M. S., DeMoss J. A. Role of alternative promoter elements in transcription from the nar promoter of Escherichia coli. J Bacteriol. 1992 Feb;174(4):1119–1123. doi: 10.1128/jb.174.4.1119-1123.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock G. M., Berman M. L., Silhavy T. J. Chimeric genetics with beta-galactosidase. Gene Amplif Anal. 1983;3:27–64. [PubMed] [Google Scholar]
- Weisemann J. M., Funk C., Weinstock G. M. Measurement of in vivo expression of the recA gene of Escherichia coli by using lacZ gene fusions. J Bacteriol. 1984 Oct;160(1):112–121. doi: 10.1128/jb.160.1.112-121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisemann J. M., Weinstock G. M. Direct selection of mutations reducing transcription or translation of the recA gene of Escherichia coli with a recA-lacZ protein fusion. J Bacteriol. 1985 Aug;163(2):748–755. doi: 10.1128/jb.163.2.748-755.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. P., Faro A., Zubay G. Mitomycin-induced lethality of Escherichia coli cells containing the ColE1 Plasmid: involvement of the kil gene. J Bacteriol. 1985 Jul;163(1):174–179. doi: 10.1128/jb.163.1.174-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. P., Yan L. F., Zubay G. Regulation of gene expression in plasmid ColE1: delayed expression of the kil gene. J Bacteriol. 1988 Dec;170(12):5460–5467. doi: 10.1128/jb.170.12.5460-5467.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crombrugghe B., Busby S., Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984 May 25;224(4651):831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]