Abstract
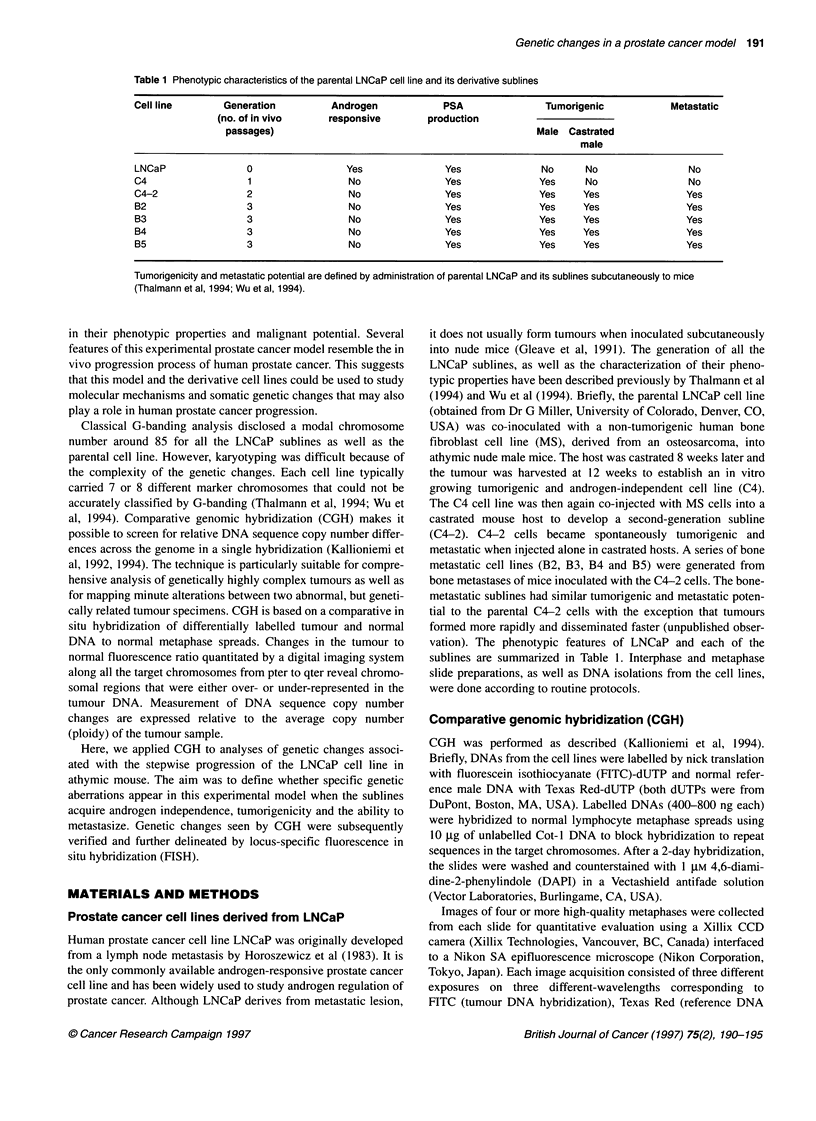
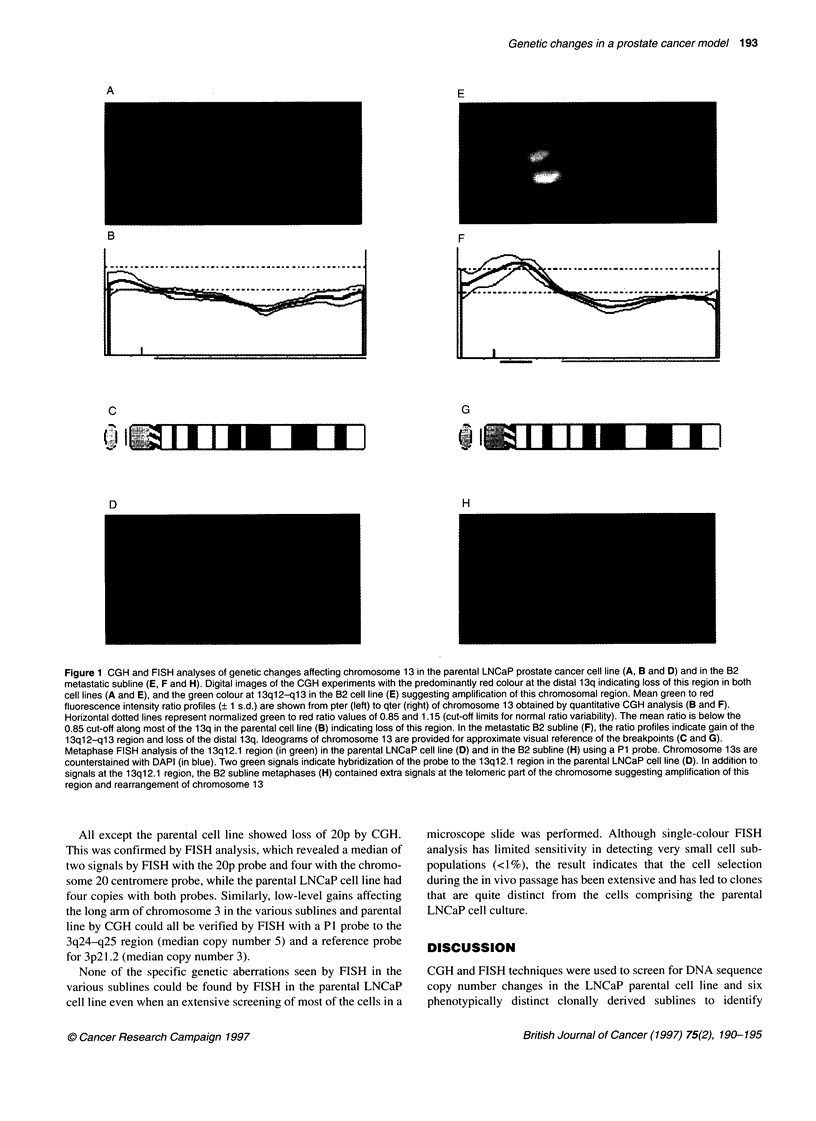
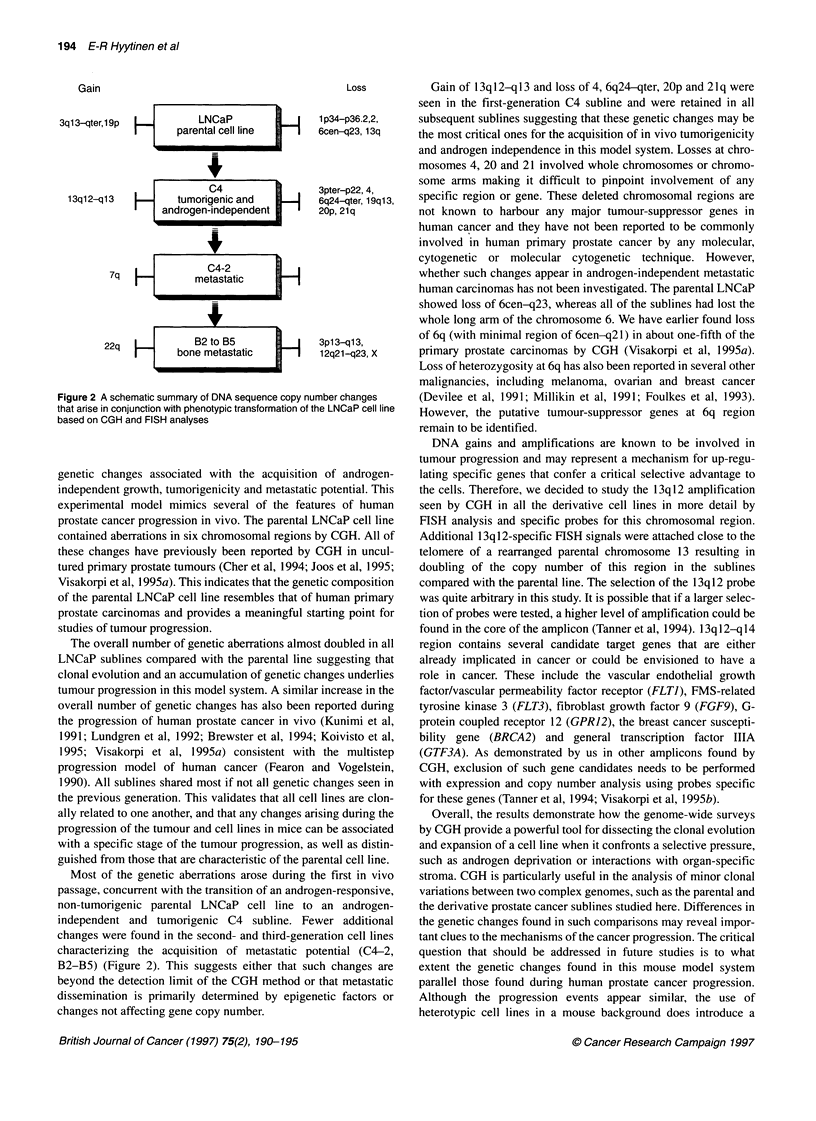
Genetic changes underlying the progression of human prostate cancer are incompletely understood. Recently, an experimental model system that resembles human prostate cancer progression was developed based on the serial passage of an androgen-responsive, non-tumorigenic LNCaP prostate cancer cell line into athymic castrated mice. Six different sublines, derived after one, two or three rounds of in vivo passage, sequentially acquired androgen independence and tumorigenicity as well as metastatic capacity. Here, we used comparative genomic hybridization (CGH) and locus-specific fluorescence in situ hybridization (FISH) analysis to search for genetic changes that may underlie the phenotypic progression events in this model system. Six genetic aberrations were seen by CGH in the parental LNCaP cell line. The derivative sublines shared virtually all these changes, indicating a common clonal origin, but also contained 3-7 additional genetic changes. Gain of the 13q12-q13 chromosomal region as well as losses of 4, 6q24-qter, 20p and 21q were associated with androgen independence and tumorigenicity with additional changes correlating with metastasis. In conclusion, an accumulation of genetic changes correlates with tumour progression in this experimental in vivo model of prostate cancer progression. It is possible that the specific chromosomal aberrations involved in this model system may provide clues to the location of genes involved in human prostate cancer progression and metastasis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackard C. E., Byar D. P., Jordan W. P., Jr Orchiectomy for advanced prostatic carcinoma. A reevaluation. Urology. 1973 Jun;1(6):553–560. doi: 10.1016/0090-4295(73)90515-3. [DOI] [PubMed] [Google Scholar]
- Brewster S. F., Browne S., Brown K. W. Somatic allelic loss at the DCC, APC, nm23-H1 and p53 tumor suppressor gene loci in human prostatic carcinoma. J Urol. 1994 Apr;151(4):1073–1077. doi: 10.1016/s0022-5347(17)35186-8. [DOI] [PubMed] [Google Scholar]
- Cher M. L., MacGrogan D., Bookstein R., Brown J. A., Jenkins R. B., Jensen R. H. Comparative genomic hybridization, allelic imbalance, and fluorescence in situ hybridization on chromosome 8 in prostate cancer. Genes Chromosomes Cancer. 1994 Nov;11(3):153–162. doi: 10.1002/gcc.2870110304. [DOI] [PubMed] [Google Scholar]
- Devilee P., van Vliet M., van Sloun P., Kuipers Dijkshoorn N., Hermans J., Pearson P. L., Cornelisse C. J. Allelotype of human breast carcinoma: a second major site for loss of heterozygosity is on chromosome 6q. Oncogene. 1991 Sep;6(9):1705–1711. [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Foulkes W. D., Ragoussis J., Stamp G. W., Allan G. J., Trowsdale J. Frequent loss of heterozygosity on chromosome 6 in human ovarian carcinoma. Br J Cancer. 1993 Mar;67(3):551–559. doi: 10.1038/bjc.1993.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibas Z., Becher R., Kawinski E., Horoszewicz J., Sandberg A. A. A high-resolution study of chromosome changes in a human prostatic carcinoma cell line (LNCaP). Cancer Genet Cytogenet. 1984 Apr;11(4):399–404. doi: 10.1016/0165-4608(84)90020-7. [DOI] [PubMed] [Google Scholar]
- Gittes R. F. Carcinoma of the prostate. N Engl J Med. 1991 Jan 24;324(4):236–245. doi: 10.1056/NEJM199101243240406. [DOI] [PubMed] [Google Scholar]
- Horoszewicz J. S., Leong S. S., Kawinski E., Karr J. P., Rosenthal H., Chu T. M., Mirand E. A., Murphy G. P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983 Apr;43(4):1809–1818. [PubMed] [Google Scholar]
- Joos S., Bergerheim U. S., Pan Y., Matsuyama H., Bentz M., du Manoir S., Lichter P. Mapping of chromosomal gains and losses in prostate cancer by comparative genomic hybridization. Genes Chromosomes Cancer. 1995 Dec;14(4):267–276. doi: 10.1002/gcc.2870140405. [DOI] [PubMed] [Google Scholar]
- Kaighn M. E., Narayan K. S., Ohnuki Y., Lechner J. F., Jones L. W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol. 1979 Jul;17(1):16–23. [PubMed] [Google Scholar]
- Kallioniemi A., Kallioniemi O. P., Sudar D., Rutovitz D., Gray J. W., Waldman F., Pinkel D. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science. 1992 Oct 30;258(5083):818–821. doi: 10.1126/science.1359641. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O. P., Kallioniemi A., Piper J., Isola J., Waldman F. M., Gray J. W., Pinkel D. Optimizing comparative genomic hybridization for analysis of DNA sequence copy number changes in solid tumors. Genes Chromosomes Cancer. 1994 Aug;10(4):231–243. doi: 10.1002/gcc.2870100403. [DOI] [PubMed] [Google Scholar]
- Lundgren R., Heim S., Mandahl N., Anderson H., Mitelman F. Chromosome abnormalities are associated with unfavorable outcome in prostatic cancer patients. J Urol. 1992 Mar;147(3 Pt 2):784–788. doi: 10.1016/s0022-5347(17)37386-x. [DOI] [PubMed] [Google Scholar]
- Millikin D., Meese E., Vogelstein B., Witkowski C., Trent J. Loss of heterozygosity for loci on the long arm of chromosome 6 in human malignant melanoma. Cancer Res. 1991 Oct 15;51(20):5449–5453. [PubMed] [Google Scholar]
- Piper J., Rutovitz D., Sudar D., Kallioniemi A., Kallioniemi O. P., Waldman F. M., Gray J. W., Pinkel D. Computer image analysis of comparative genomic hybridization. Cytometry. 1995 Jan 1;19(1):10–26. doi: 10.1002/cyto.990190104. [DOI] [PubMed] [Google Scholar]
- Stone K. R., Mickey D. D., Wunderli H., Mickey G. H., Paulson D. F. Isolation of a human prostate carcinoma cell line (DU 145). Int J Cancer. 1978 Mar 15;21(3):274–281. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
- Tanner M. M., Tirkkonen M., Kallioniemi A., Collins C., Stokke T., Karhu R., Kowbel D., Shadravan F., Hintz M., Kuo W. L. Increased copy number at 20q13 in breast cancer: defining the critical region and exclusion of candidate genes. Cancer Res. 1994 Aug 15;54(16):4257–4260. [PubMed] [Google Scholar]
- Thalmann G. N., Anezinis P. E., Chang S. M., Zhau H. E., Kim E. E., Hopwood V. L., Pathak S., von Eschenbach A. C., Chung L. W. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994 May 15;54(10):2577–2581. [PubMed] [Google Scholar]
- Visakorpi T., Hyytinen E., Koivisto P., Tanner M., Keinänen R., Palmberg C., Palotie A., Tammela T., Isola J., Kallioniemi O. P. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995 Apr;9(4):401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- Wingo P. A., Tong T., Bolden S. Cancer statistics, 1995. CA Cancer J Clin. 1995 Jan-Feb;45(1):8–30. doi: 10.3322/canjclin.45.1.8. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Hsieh J. T., Gleave M. E., Brown N. M., Pathak S., Chung L. W. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer. 1994 May 1;57(3):406–412. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]