Abstract
5'-Deoxy-5-fluorouridine (5'-DFUR) and 1-(tetrahydro-2-furyl)-5-fluorouracil (tegafur), prodrugs of 5-fluorouracil (5-FU), are anticancer agents activated by thymidine phosphorylase (dThdPase). As it is well known that the levels of dThdPase are higher in tumours than in normal tissue, it should be advantageous to use such pyrimidine antimetabolites for the selective inhibition of tumour growth. However, tumours are not necessarily sensitive to 5'-DFUR and tegafur because their levels of dThdPase vary. In this study, we examined whether transfection of tumour cells with a human platelet-derived endothelial cell growth factor (PD-ECGF) complementary DNA (cDNA) expressing dThdPase would sensitize the cells to the cytotoxic effects of pyrimidine antimetabolites in vitro. A cDNA encoding PD-ECGF was transfected into PC-9 cells (human lung adenocarcinoma). The transfected cells, PC9-DPE2, had a more than 50 times higher activity of dThdPase than the parental PC-9 cells or control PC-9 cells transfected with the pcDNA3 vector alone (PC9-D1). They were more sensitive than parental PC-9 or PC9-D1 cells not only to 5'-DFUR and tegafur but also to 5-FU. In addition, we demonstrated that PC9-DPE2 cells are able to potentiate the cytotoxic effects of 5'-DFUR towards co-cultured parental PC-9 cells. This "bystander effect' did not require cell-cell contact. These results suggest that transfection of PD-ECGF (dThdPase) genes may be useful as a gene therapy strategy for cancer treatment.
Full text
PDF

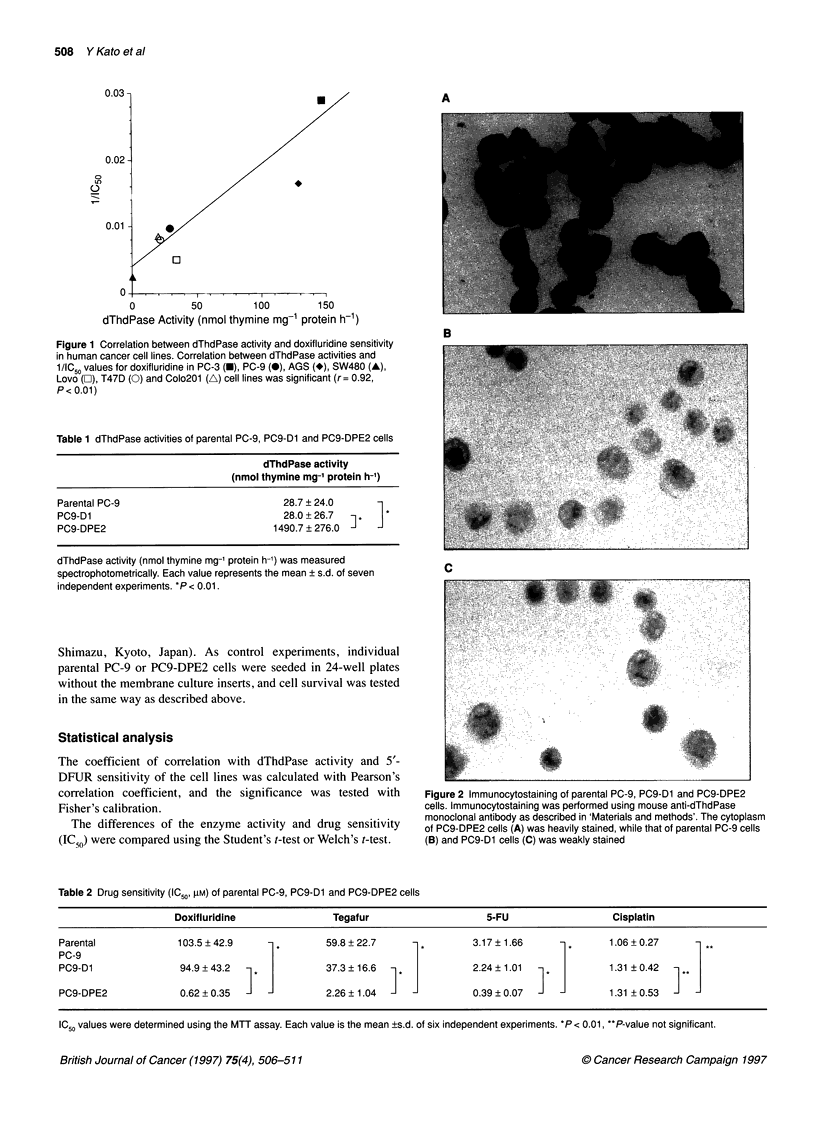
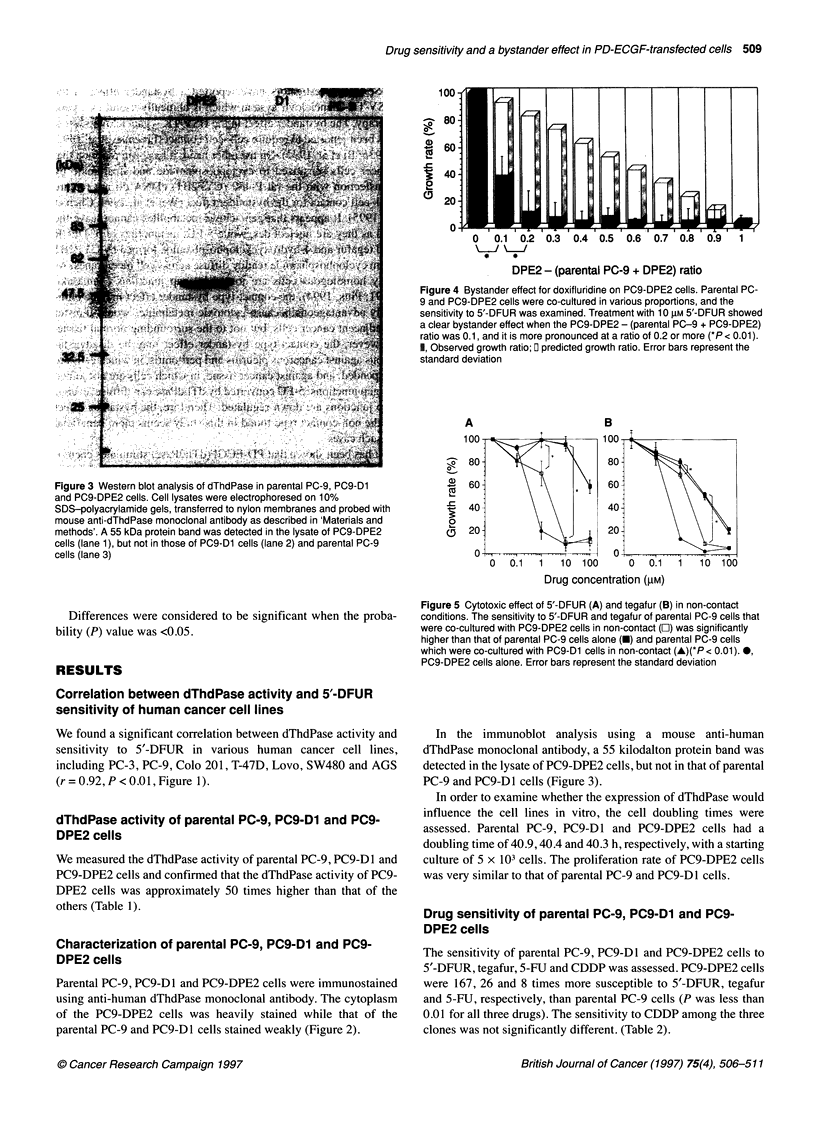


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bi W. L., Parysek L. M., Warnick R., Stambrook P. J. In vitro evidence that metabolic cooperation is responsible for the bystander effect observed with HSV tk retroviral gene therapy. Hum Gene Ther. 1993 Dec;4(6):725–731. doi: 10.1089/hum.1993.4.6-725. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen L., Waxman D. J. Intratumoral activation and enhanced chemotherapeutic effect of oxazaphosphorines following cytochrome P-450 gene transfer: development of a combined chemotherapy/cancer gene therapy strategy. Cancer Res. 1995 Feb 1;55(3):581–589. [PubMed] [Google Scholar]
- Cook A. F., Holman M. J., Kramer M. J., Trown P. W. Fluorinated pyrimidine nucleosides. 3. Synthesis and antitumor activity of a series of 5'-deoxy-5-fluoropyrimidine nucleosides. J Med Chem. 1979 Nov;22(11):1330–1335. doi: 10.1021/jm00197a010. [DOI] [PubMed] [Google Scholar]
- Eda H., Fujimoto K., Watanabe S., Ura M., Hino A., Tanaka Y., Wada K., Ishitsuka H. Cytokines induce thymidine phosphorylase expression in tumor cells and make them more susceptible to 5'-deoxy-5-fluorouridine. Cancer Chemother Pharmacol. 1993;32(5):333–338. doi: 10.1007/BF00735915. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Ellis L. M. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994 Oct 21;79(2):185–188. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Finnis C., Dodsworth N., Pollitt C. E., Carr G., Sleep D. Thymidine phosphorylase activity of platelet-derived endothelial cell growth factor is responsible for endothelial cell mitogenicity. Eur J Biochem. 1993 Feb 15;212(1):201–210. doi: 10.1111/j.1432-1033.1993.tb17651.x. [DOI] [PubMed] [Google Scholar]
- Freeman S. M., Abboud C. N., Whartenby K. A., Packman C. H., Koeplin D. S., Moolten F. L., Abraham G. N. The "bystander effect": tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993 Nov 1;53(21):5274–5283. [PubMed] [Google Scholar]
- Fujita H., Ogawa K., Nakagawa H., Kawaguchi K., Nakagawa Y., Doi Y. [Pharmacokinetics of 5'-deoxy-5-fluorouridine (5'-DFUR)]. Nihon Gan Chiryo Gakkai Shi. 1983 Jun 20;18(4):916–926. [PubMed] [Google Scholar]
- Furukawa T., Yoshimura A., Sumizawa T., Haraguchi M., Akiyama S., Fukui K., Ishizawa M., Yamada Y. Angiogenic factor. Nature. 1992 Apr 23;356(6371):668–668. doi: 10.1038/356668a0. [DOI] [PubMed] [Google Scholar]
- Haraguchi M., Furukawa T., Sumizawa T., Akiyama S. Sensitivity of human KB cells expressing platelet-derived endothelial cell growth factor to pyrimidine antimetabolites. Cancer Res. 1993 Dec 1;53(23):5680–5682. [PubMed] [Google Scholar]
- Heldin N. E., Usuki K., Bergh J., Westermark B., Heldin C. H. Differential expression of platelet-derived endothelial cell growth factor/thymidine phosphorylase in human lung carcinoma cell lines. Br J Cancer. 1993 Oct;68(4):708–711. doi: 10.1038/bjc.1993.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa F., Miyazono K., Hellman U., Drexler H., Wernstedt C., Hagiwara K., Usuki K., Takaku F., Risau W., Heldin C. H. Identification of angiogenic activity and the cloning and expression of platelet-derived endothelial cell growth factor. Nature. 1989 Apr 13;338(6216):557–562. doi: 10.1038/338557a0. [DOI] [PubMed] [Google Scholar]
- Kono A., Hara Y., Sugata S., Karube Y., Matsushima Y., Ishitsuka H. Activation of 5'-deoxy-5-fluorouridine by thymidine phosphorylase in human tumors. Chem Pharm Bull (Tokyo) 1983 Jan;31(1):175–178. doi: 10.1248/cpb.31.175. [DOI] [PubMed] [Google Scholar]
- Miwa M., Nishimura J., Kamiyama T., Ishitsuka H. [Conversion of 5'-deoxy-5-fluorouridine to 5-FU by pyrimidine nucleoside phosphorylases in normal and tumor tissues from rodents bearing tumors and cancer patients]. Gan To Kagaku Ryoho. 1987 Oct;14(10):2924–2929. [PubMed] [Google Scholar]
- Miyadera K., Sumizawa T., Haraguchi M., Yoshida H., Konstanty W., Yamada Y., Akiyama S. Role of thymidine phosphorylase activity in the angiogenic effect of platelet derived endothelial cell growth factor/thymidine phosphorylase. Cancer Res. 1995 Apr 15;55(8):1687–1690. [PubMed] [Google Scholar]
- Miyazono K., Okabe T., Urabe A., Takaku F., Heldin C. H. Purification and properties of an endothelial cell growth factor from human platelets. J Biol Chem. 1987 Mar 25;262(9):4098–4103. [PubMed] [Google Scholar]
- Moghaddam A., Bicknell R. Expression of platelet-derived endothelial cell growth factor in Escherichia coli and confirmation of its thymidine phosphorylase activity. Biochemistry. 1992 Dec 8;31(48):12141–12146. doi: 10.1021/bi00163a024. [DOI] [PubMed] [Google Scholar]
- Moghaddam A., Zhang H. T., Fan T. P., Hu D. E., Lees V. C., Turley H., Fox S. B., Gatter K. C., Harris A. L., Bicknell R. Thymidine phosphorylase is angiogenic and promotes tumor growth. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):998–1002. doi: 10.1073/pnas.92.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolten F. L., Wells J. M. Curability of tumors bearing herpes thymidine kinase genes transferred by retroviral vectors. J Natl Cancer Inst. 1990 Feb 21;82(4):297–300. doi: 10.1093/jnci/82.4.297. [DOI] [PubMed] [Google Scholar]
- Oldfield E. H., Ram Z., Culver K. W., Blaese R. M., DeVroom H. L., Anderson W. F. Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir. Hum Gene Ther. 1993 Feb;4(1):39–69. doi: 10.1089/hum.1993.4.1-39. [DOI] [PubMed] [Google Scholar]
- Patterson A. V., Zhang H., Moghaddam A., Bicknell R., Talbot D. C., Stratford I. J., Harris A. L. Increased sensitivity to the prodrug 5'-deoxy-5-fluorouridine and modulation of 5-fluoro-2'-deoxyuridine sensitivity in MCF-7 cells transfected with thymidine phosphorylase. Br J Cancer. 1995 Sep;72(3):669–675. doi: 10.1038/bjc.1995.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts J. D. Cancer gene therapy: a bystander effect using the gap junctional pathway. Mol Carcinog. 1994 Nov;11(3):127–130. doi: 10.1002/mc.2940110302. [DOI] [PubMed] [Google Scholar]
- Sumizawa T., Furukawa T., Haraguchi M., Yoshimura A., Takeyasu A., Ishizawa M., Yamada Y., Akiyama S. Thymidine phosphorylase activity associated with platelet-derived endothelial cell growth factor. J Biochem. 1993 Jul;114(1):9–14. doi: 10.1093/oxfordjournals.jbchem.a124146. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Hongu Y., Fukazawa H., Ichihara S., Shimizu H. Tissue distribution of 5'-deoxy-5-fluorouridine and derived 5-fluorouracil in tumor-bearing mice and rats. Gan. 1980 Apr;71(2):238–245. [PubMed] [Google Scholar]
- Toi M., Hoshina S., Taniguchi T., Yamamoto Y., Ishitsuka H., Tominaga T. Expression of platelet-derived endothelial cell growth factor/thymidine phosphorylase in human breast cancer. Int J Cancer. 1995 Apr 21;64(2):79–82. doi: 10.1002/ijc.2910640202. [DOI] [PubMed] [Google Scholar]
- Usuki K., Saras J., Waltenberger J., Miyazono K., Pierce G., Thomason A., Heldin C. H. Platelet-derived endothelial cell growth factor has thymidine phosphorylase activity. Biochem Biophys Res Commun. 1992 May 15;184(3):1311–1316. doi: 10.1016/s0006-291x(05)80025-7. [DOI] [PubMed] [Google Scholar]
- Wei M. X., Tamiya T., Chase M., Boviatsis E. J., Chang T. K., Kowall N. W., Hochberg F. H., Waxman D. J., Breakefield X. O., Chiocca E. A. Experimental tumor therapy in mice using the cyclophosphamide-activating cytochrome P450 2B1 gene. Hum Gene Ther. 1994 Aug;5(8):969–978. doi: 10.1089/hum.1994.5.8-969. [DOI] [PubMed] [Google Scholar]
- Yamasaki H. Aberrant expression and function of gap junctions during carcinogenesis. Environ Health Perspect. 1991 Jun;93:191–197. doi: 10.1289/ehp.9193191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Kuwazuru Y., Furukawa T., Yoshida H., Yamada K., Akiyama S. Purification and tissue distribution of human thymidine phosphorylase; high expression in lymphocytes, reticulocytes and tumors. Biochim Biophys Acta. 1990 Apr 23;1034(1):107–113. doi: 10.1016/0304-4165(90)90160-x. [DOI] [PubMed] [Google Scholar]
- ZIMMERMAN M., SEIDENBERG J. DEOXYRIBOSYL TRANSFER. I. THYMIDINE PHOSPHORYLASE AND NUCLEOSIDE DEOXYRIBOSYLTRANSFERASE IN NORMAL AND MALIGNANT TISSUES. J Biol Chem. 1964 Aug;239:2618–2621. [PubMed] [Google Scholar]