Abstract
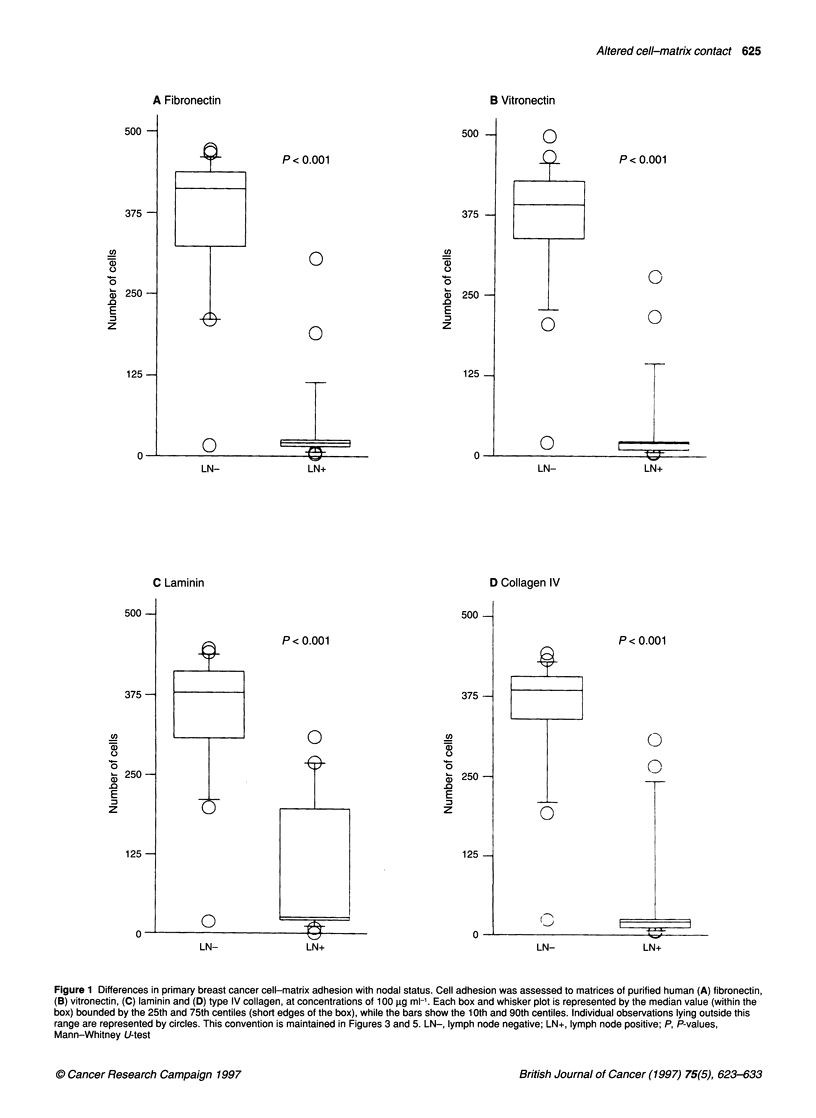
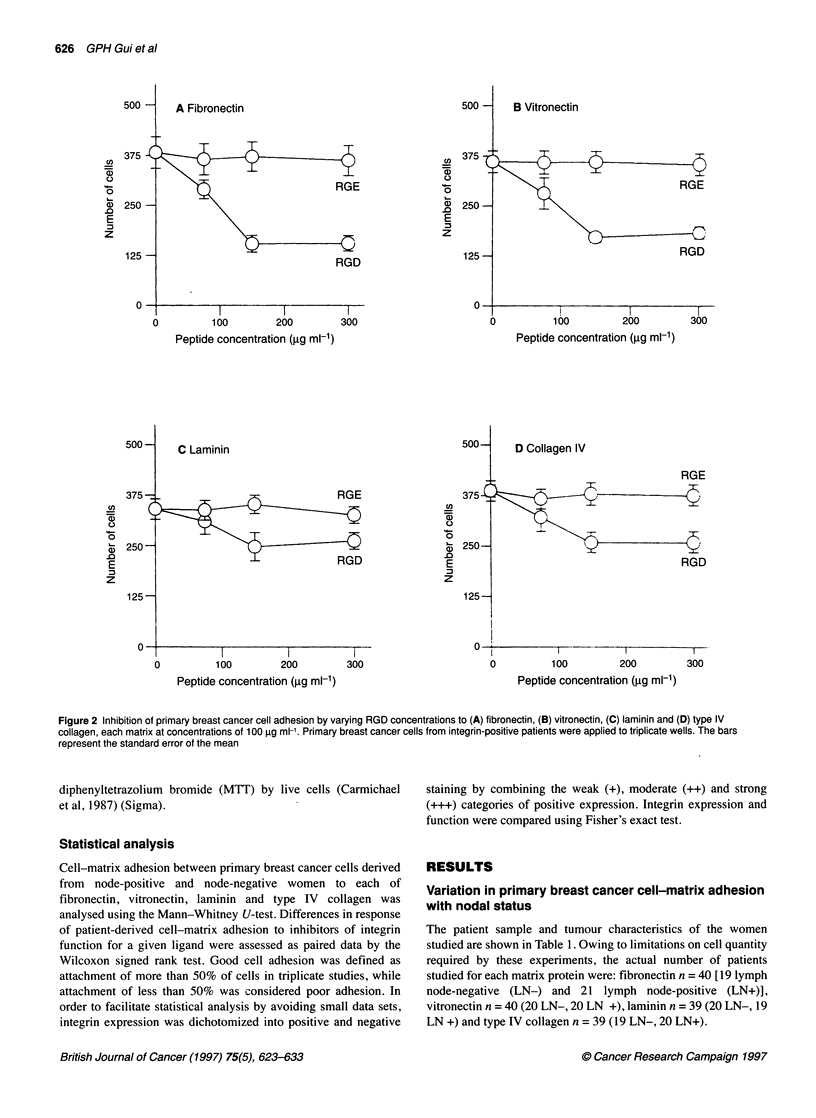
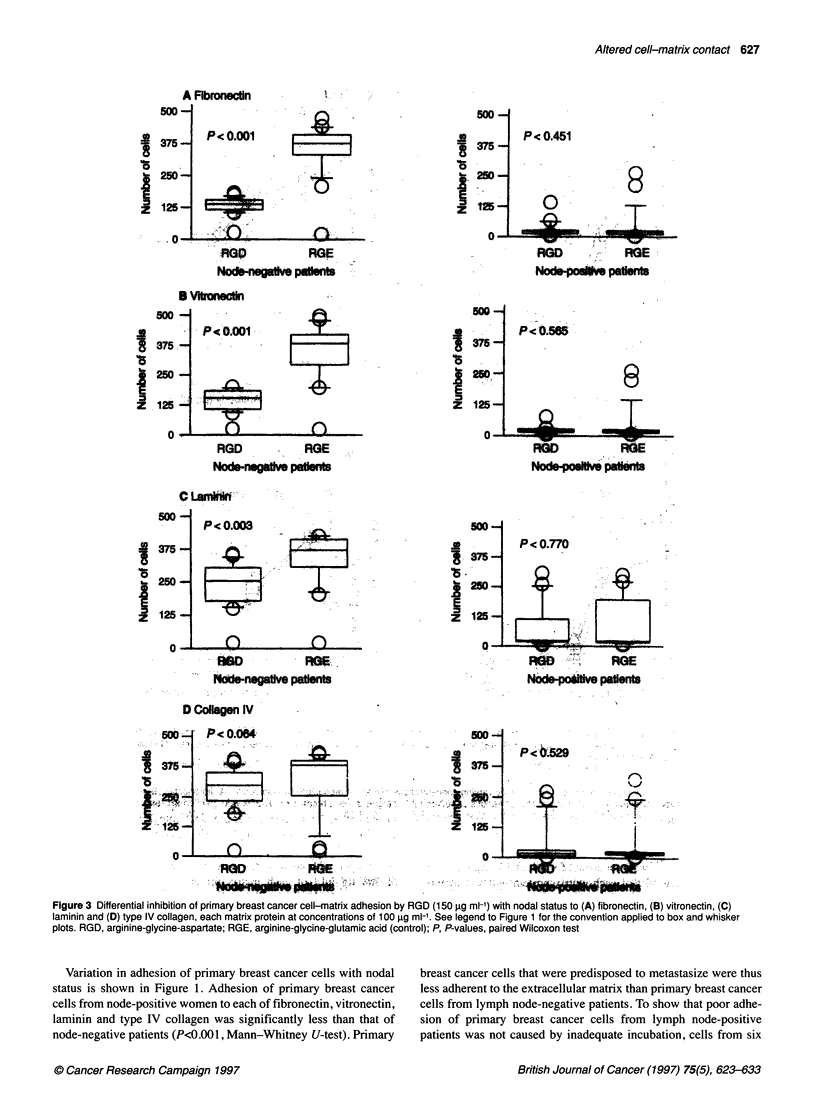
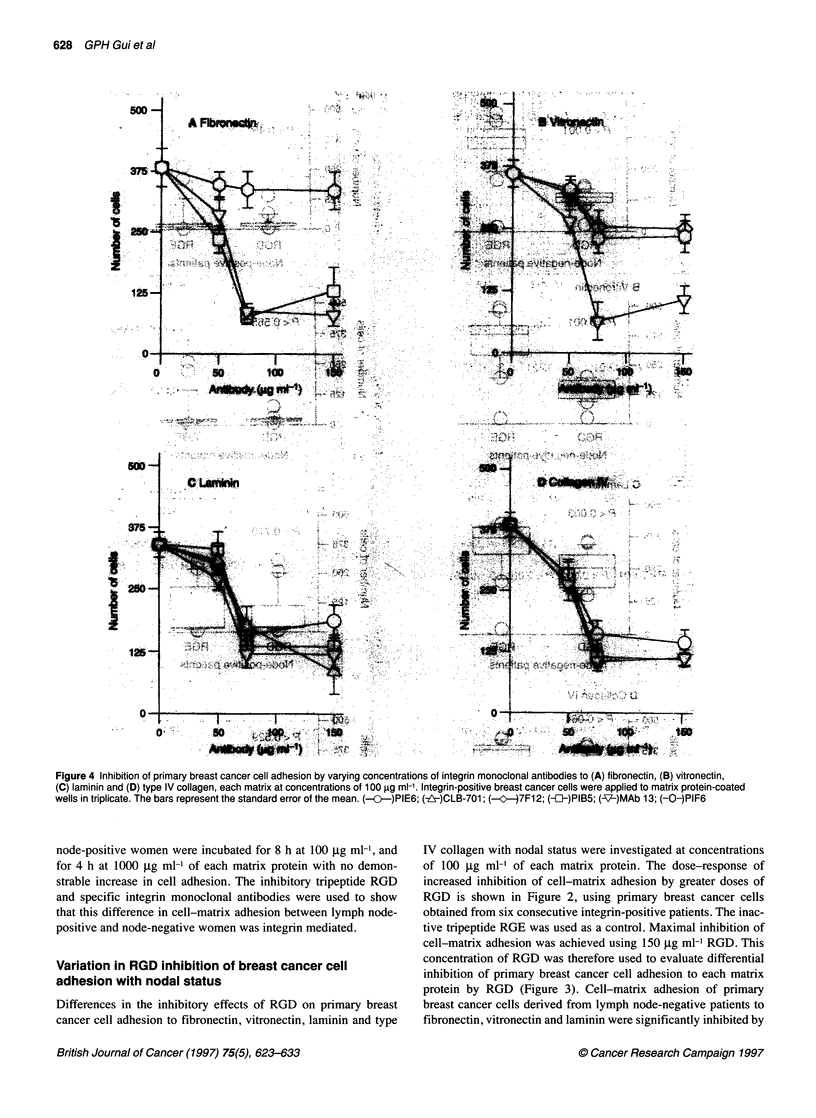
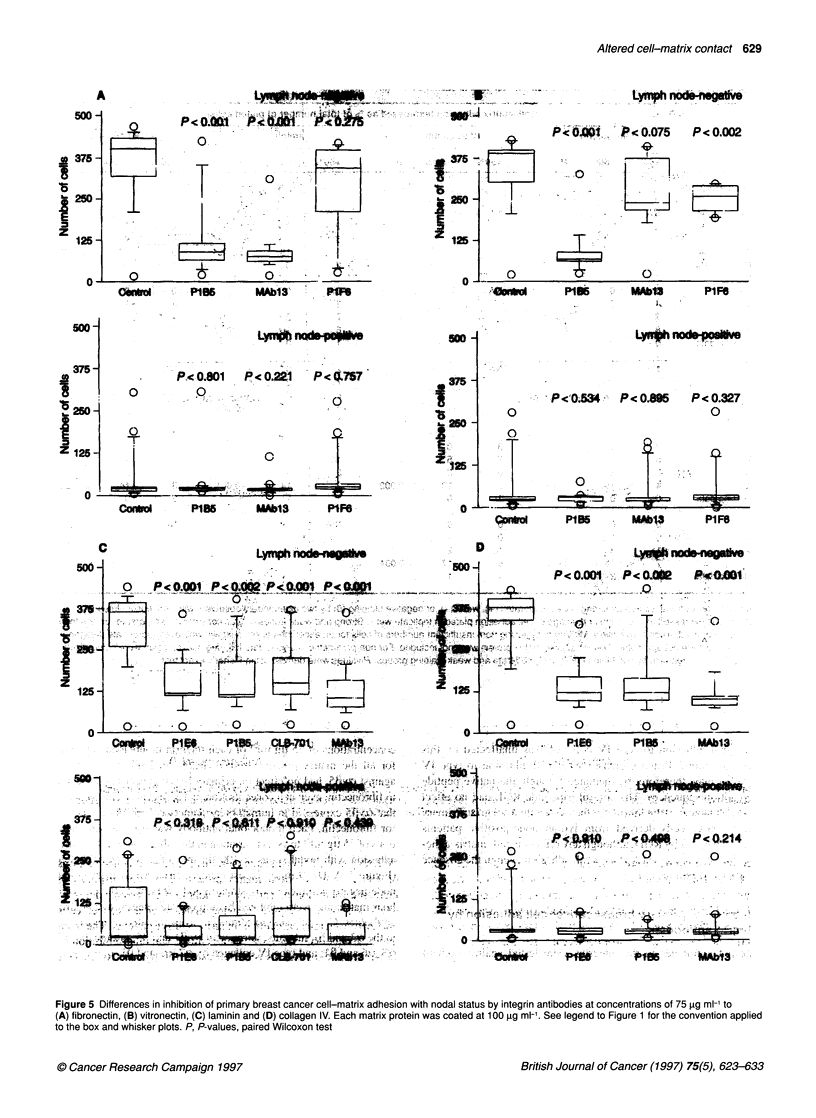
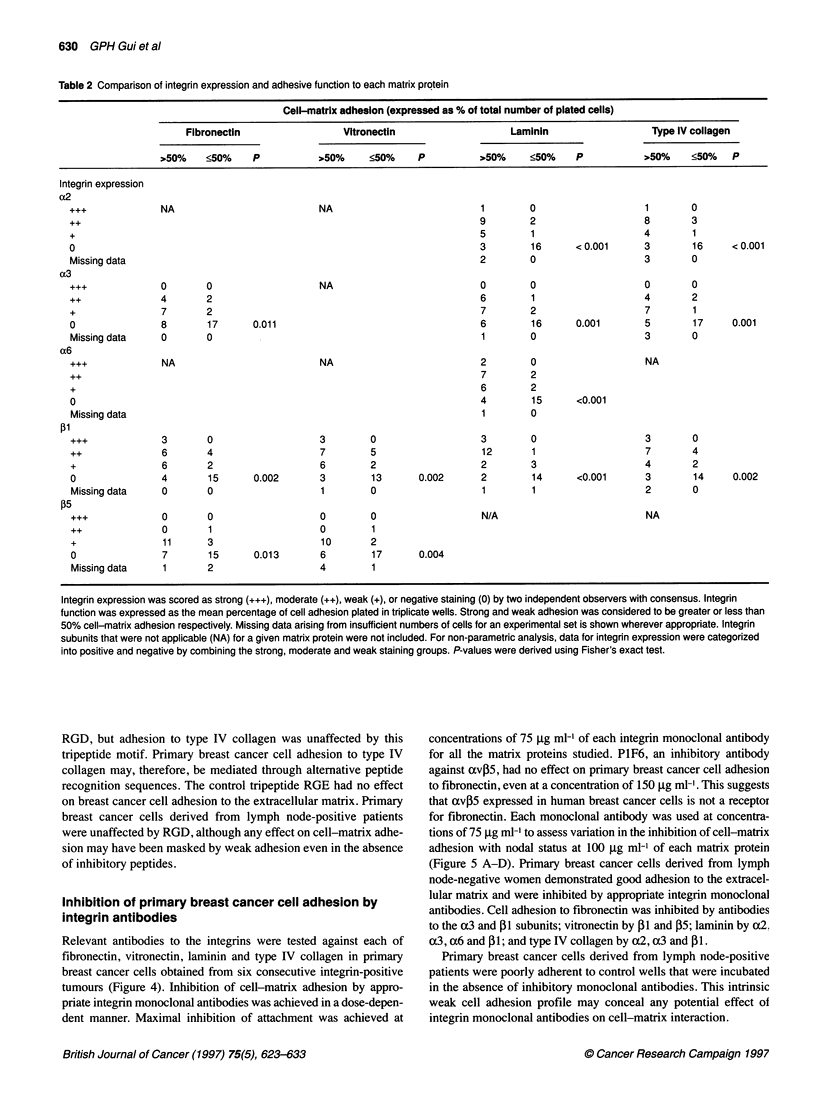
The integrins are receptors that regulate interaction between epithelial cells and the extracellular matrix. Previous studies have shown that a reduction in the expression of the alpha2beta1, alpha3beta1, alpha6beta1, alpha(v)beta1 and alpha(v)beta5 integrins in primary breast cancer is associated with positive nodal status. In order to assess the functional significance of altered integrin expression, primary breast cancer cells were derived from individual patients with known tumour characteristics using immunomagnetic separation. Purified human fibronectin, vitronectin, laminin and type IV collagen were used to represent the principal extracellular matrix proteins in an in vitro adhesion assay. Primary breast cancer cells from lymph node-positive patients were significantly less adhesive to each of the matrix proteins studied (P<0.001, Mann-Whitney U-test). Matrix adhesion of primary breast cancer cells from node-negative patients was inhibited by appropriate integrin monoclonal antibodies (P<0.001, paired Wilcoxon test). Adhesion to fibronectin, vitronectin and laminin, but not type IV collagen, was influenced by the inhibitor arginine-glycine-aspartate, suggesting that breast cancer cell recognition of collagen IV is mediated through alternative epitopes. Weak matrix adhesion correlated with loss of integrin expression in tissue sections from corresponding patients assessed using immunohistochemistry. This study demonstrates a link between altered integrin expression and function in primary breast cancers predisposed to metastasize.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Aznavoorian S., Stracke M. L., Krutzsch H., Schiffmann E., Liotta L. A. Signal transduction for chemotaxis and haptotaxis by matrix molecules in tumor cells. J Cell Biol. 1990 Apr;110(4):1427–1438. doi: 10.1083/jcb.110.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Burridge K., Turner C. E., Romer L. H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992 Nov;119(4):893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of radiosensitivity. Cancer Res. 1987 Feb 15;47(4):943–946. [PubMed] [Google Scholar]
- D'Souza B., Berdichevsky F., Kyprianou N., Taylor-Papadimitriou J. Collagen-induced morphogenesis and expression of the alpha 2-integrin subunit is inhibited in c-erbB2-transfected human mammary epithelial cells. Oncogene. 1993 Jul;8(7):1797–1806. [PubMed] [Google Scholar]
- Duband J. L., Rocher S., Chen W. T., Yamada K. M., Thiery J. P. Cell adhesion and migration in the early vertebrate embryo: location and possible role of the putative fibronectin receptor complex. J Cell Biol. 1986 Jan;102(1):160–178. doi: 10.1083/jcb.102.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990 Mar 9;60(5):849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Gui G. P., Puddefoot J. R., Vinson G. P., Wells C. A., Carpenter R. Modulation of very late activation-2 laminin receptor function in breast cancer metastasis. Surgery. 1995 Aug;118(2):245–250. doi: 10.1016/s0039-6060(05)80330-7. [DOI] [PubMed] [Google Scholar]
- Gui G. P., Wells C. A., Browne P. D., Yeomans P., Jordan S., Puddefoot J. R., Vinson G. P., Carpenter R. Integrin expression in primary breast cancer and its relation to axillary nodal status. Surgery. 1995 Jan;117(1):102–108. doi: 10.1016/s0039-6060(05)80236-3. [DOI] [PubMed] [Google Scholar]
- Hall D. E., Reichardt L. F., Crowley E., Holley B., Moezzi H., Sonnenberg A., Damsky C. H. The alpha 1/beta 1 and alpha 6/beta 1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol. 1990 Jun;110(6):2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart I. R., Saini A. Biology of tumour metastasis. Lancet. 1992 Jun 13;339(8807):1453–1457. doi: 10.1016/0140-6736(92)92039-i. [DOI] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Buck C., Beckerle M. C., Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986 Apr 10;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Humphries M. J., Komoriya A., Akiyama S. K., Olden K., Yamada K. M. Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J Biol Chem. 1987 May 15;262(14):6886–6892. [PubMed] [Google Scholar]
- Humphries M. J. The molecular basis and specificity of integrin-ligand interactions. J Cell Sci. 1990 Dec;97(Pt 4):585–592. doi: 10.1242/jcs.97.4.585. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ishii M., Vroman B., LaRusso N. F. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology. 1989 Nov;97(5):1236–1247. doi: 10.1016/0016-5085(89)91695-8. [DOI] [PubMed] [Google Scholar]
- Koopman G., van Kooyk Y., de Graaff M., Meyer C. J., Figdor C. G., Pals S. T. Triggering of the CD44 antigen on T lymphocytes promotes T cell adhesion through the LFA-1 pathway. J Immunol. 1990 Dec 1;145(11):3589–3593. [PubMed] [Google Scholar]
- Kornberg L., Earp H. S., Parsons J. T., Schaller M., Juliano R. L. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992 Nov 25;267(33):23439–23442. [PubMed] [Google Scholar]
- Koukoulis G. K., Virtanen I., Korhonen M., Laitinen L., Quaranta V., Gould V. E. Immunohistochemical localization of integrins in the normal, hyperplastic, and neoplastic breast. Correlations with their functions as receptors and cell adhesion molecules. Am J Pathol. 1991 Oct;139(4):787–799. [PMC free article] [PubMed] [Google Scholar]
- Languino L. R., Gehlsen K. R., Wayner E., Carter W. G., Engvall E., Ruoslahti E. Endothelial cells use alpha 2 beta 1 integrin as a laminin receptor. J Cell Biol. 1989 Nov;109(5):2455–2462. doi: 10.1083/jcb.109.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Charo I. F., Parise L. V., Fitzgerald L. A. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988 Apr;71(4):831–843. [PubMed] [Google Scholar]
- Pignatelli M., Hanby A. M., Stamp G. W. Low expression of beta 1, alpha 2 and alpha 3 subunits of VLA integrins in malignant mammary tumours. J Pathol. 1991 Sep;165(1):25–32. doi: 10.1002/path.1711650106. [DOI] [PubMed] [Google Scholar]
- Rabinowich H., Herberman R. B., Whiteside T. L. Differential effects of IL12 and IL2 on expression and function of cellular adhesion molecules on purified human natural killer cells. Cell Immunol. 1993 Dec;152(2):481–498. doi: 10.1006/cimm.1993.1306. [DOI] [PubMed] [Google Scholar]
- Rabinowich H., Sedlmayr P., Herberman R. B., Whiteside T. L. Response of human NK cells to IL-6 alterations of the cell surface phenotype, adhesion to fibronectin and laminin, and tumor necrosis factor-alpha/beta secretion. J Immunol. 1993 Jun 1;150(11):4844–4855. [PubMed] [Google Scholar]
- Rasmussen A. M., Smeland E. B., Erikstein B. K., Caignault L., Funderud S. A new method for detachment of Dynabeads from positively selected B lymphocytes. J Immunol Methods. 1992 Feb 5;146(2):195–202. doi: 10.1016/0022-1759(92)90228-l. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. The Walter Herbert Lecture. Control of cell motility and tumour invasion by extracellular matrix interactions. Br J Cancer. 1992 Aug;66(2):239–242. doi: 10.1038/bjc.1992.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro S. A., Rajpara S. M., Staatz W. D., Woods V. L., Jr Isolation and characterization of a platelet surface collagen binding complex related to VLA-2. Biochem Biophys Res Commun. 1988 May 31;153(1):217–223. doi: 10.1016/s0006-291x(88)81211-7. [DOI] [PubMed] [Google Scholar]
- Schreiner C., Fisher M., Hussein S., Juliano R. L. Increased tumorigenicity of fibronectin receptor deficient Chinese hamster ovary cell variants. Cancer Res. 1991 Mar 15;51(6):1738–1740. [PubMed] [Google Scholar]
- Somogyi L., Lasić Z., Vukicević S., Banfić H. Collagen type IV stimulates an increase in intracellular Ca2+ in pancreatic acinar cells via activation of phospholipase C. Biochem J. 1994 May 1;299(Pt 3):603–611. doi: 10.1042/bj2990603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Peterson J. A., Arklie J., Burchell J., Ceriani R. L., Bodmer W. F. Monoclonal antibodies to epithelium-specific components of the human milk fat globule membrane: production and reaction with cells in culture. Int J Cancer. 1981 Jul 15;28(1):17–21. doi: 10.1002/ijc.2910280104. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W., Yamada S. S., Gralnick H., Chen W. T., Akiyama S. K. Monoclonal antibody and synthetic peptide inhibitors of human tumor cell migration. Cancer Res. 1990 Aug 1;50(15):4485–4496. [PubMed] [Google Scholar]
- Zutter M. M., Mazoujian G., Santoro S. A. Decreased expression of integrin adhesive protein receptors in adenocarcinoma of the breast. Am J Pathol. 1990 Oct;137(4):863–870. [PMC free article] [PubMed] [Google Scholar]



