Abstract
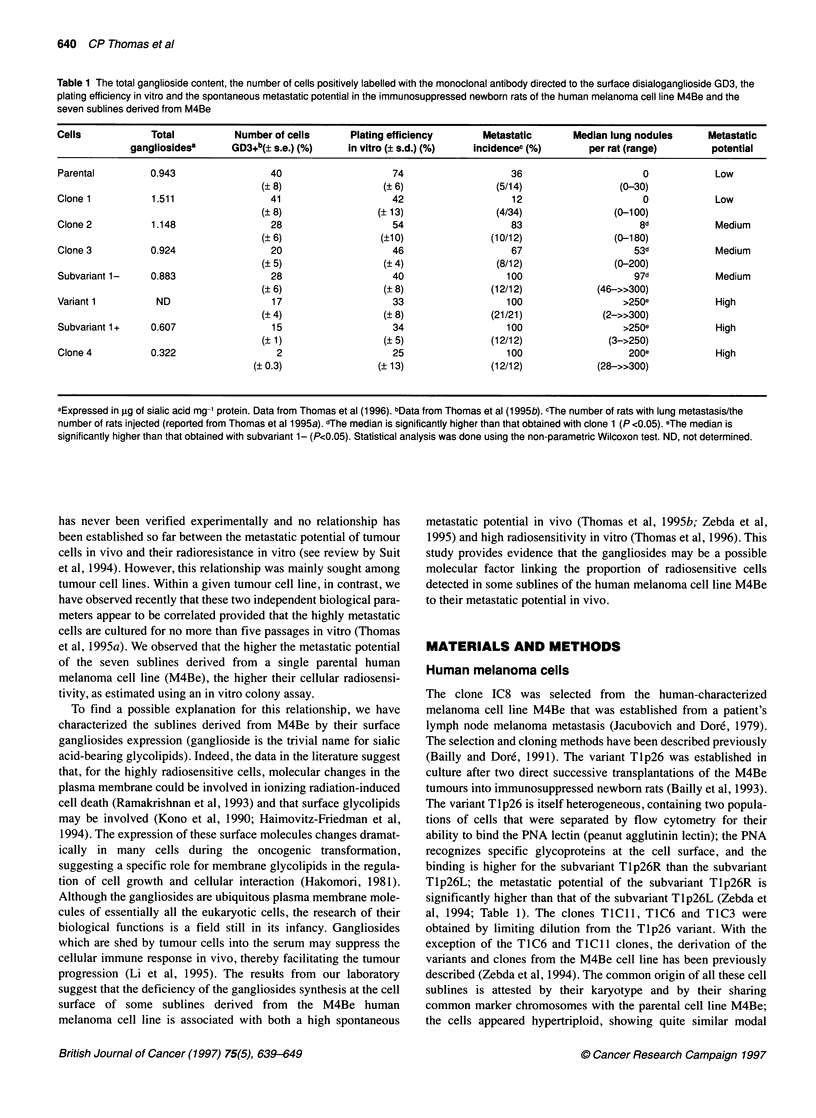
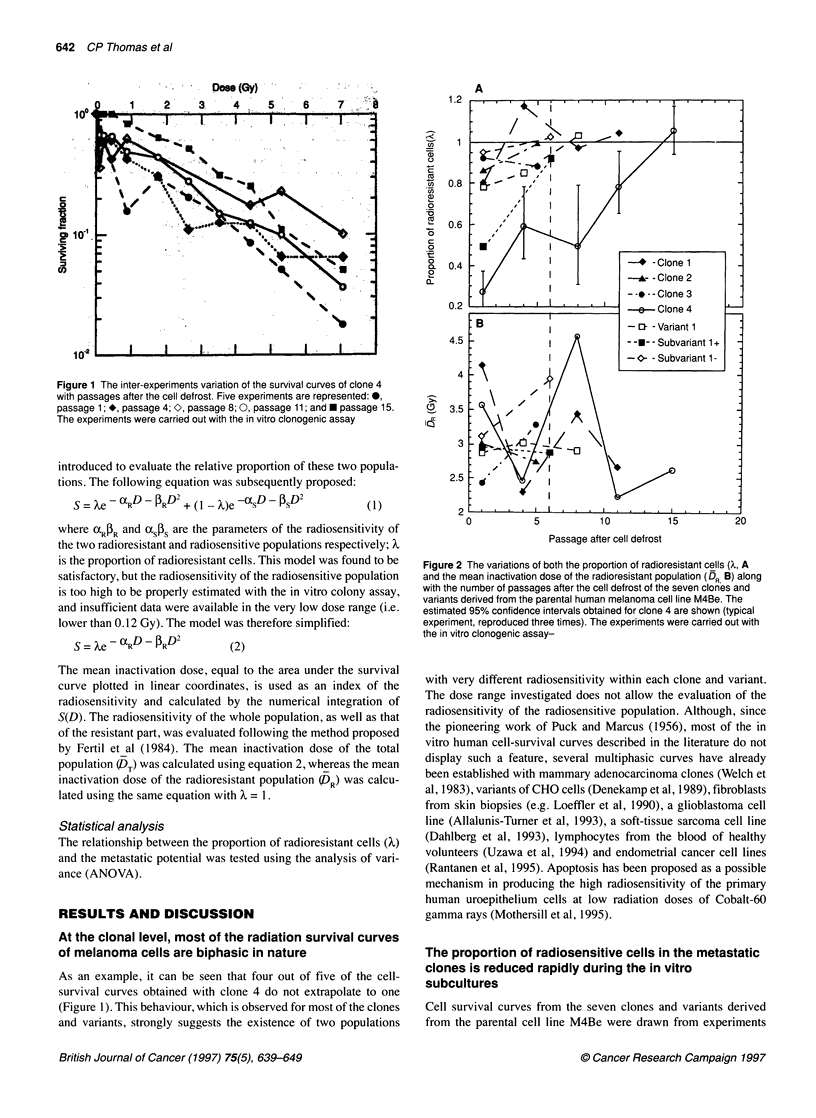
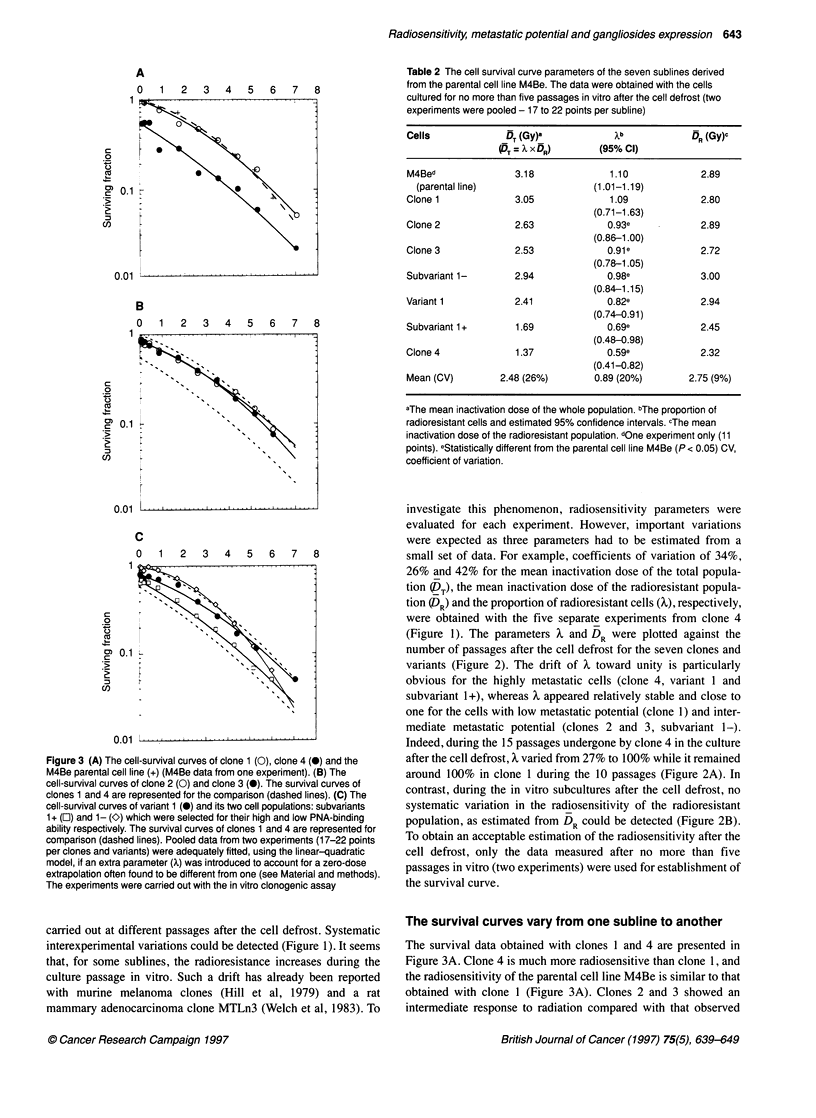
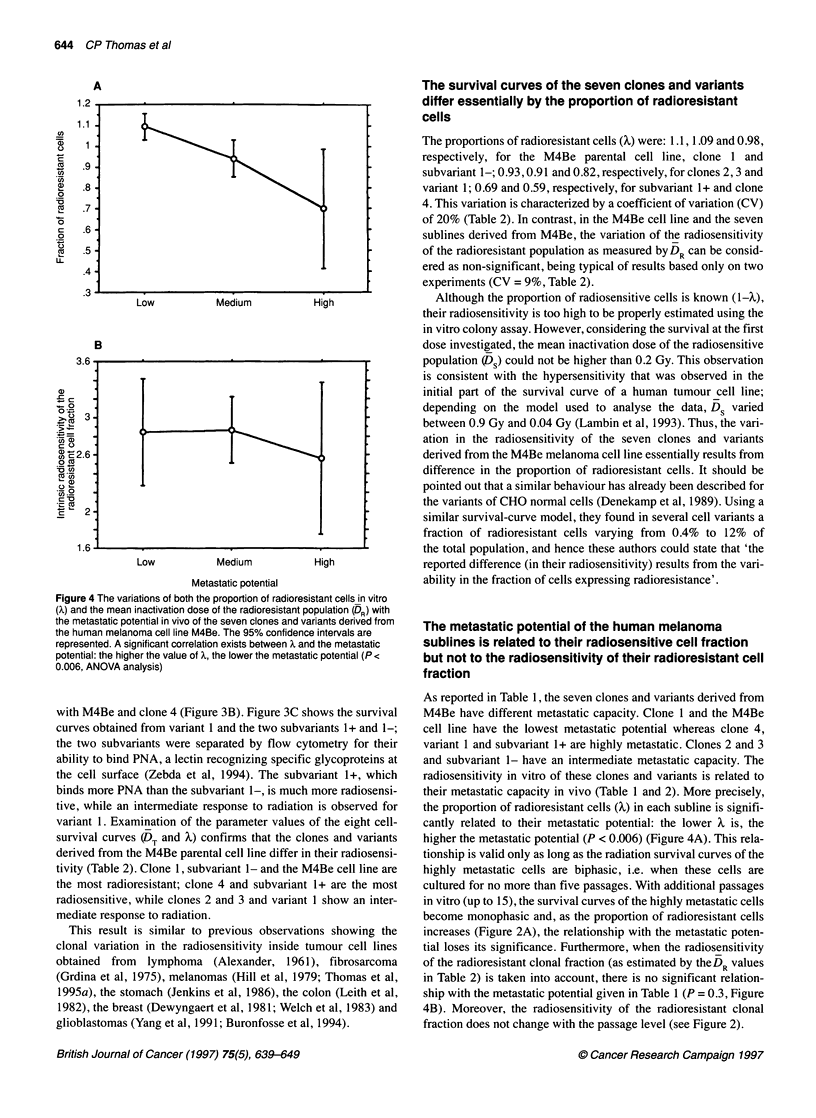
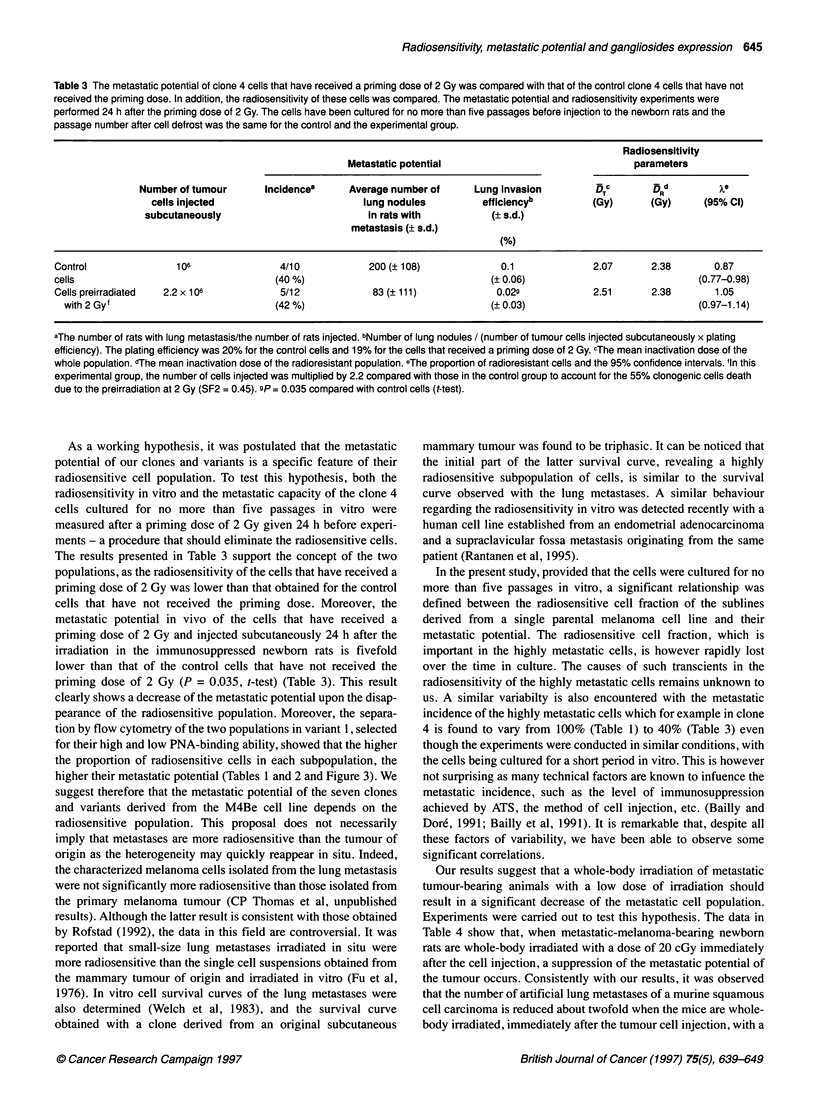
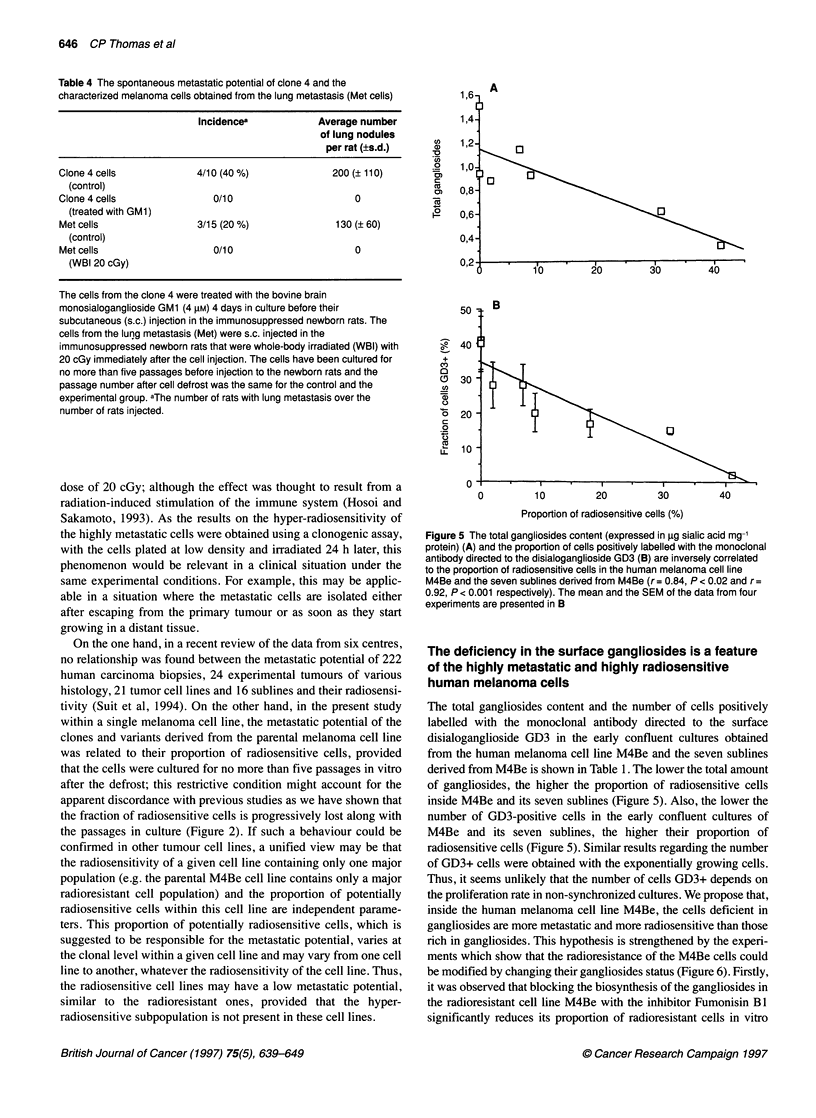
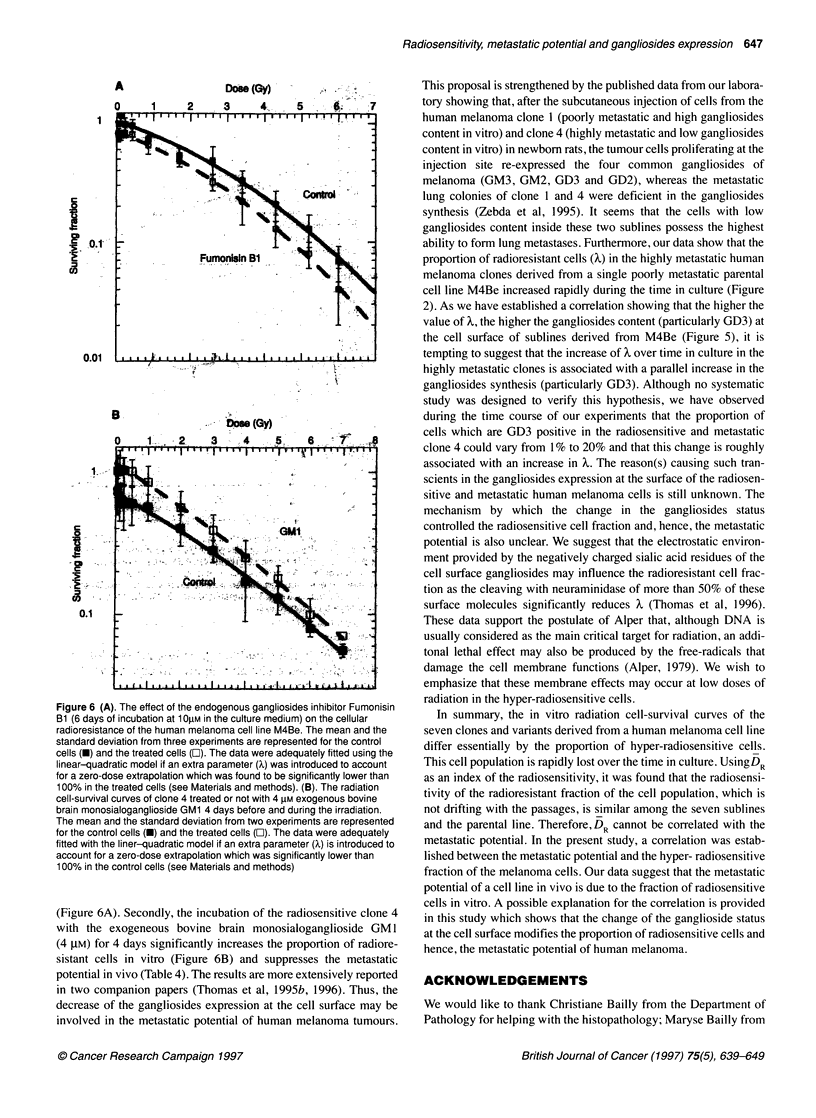
With an experimental model of spontaneous lung metastases in immunosuppressed newborn rats, seven clones and variants with different metastatic potential and gangliosides expression were derived from a single parental human melanoma cell line M4Be. The cellular radiosensitivity of M4Be and its seven sublines was estimated using an in vitro colony assay. The total amount of gangliosides in M4Be and its seven sublines was determined by cell extraction and thin-layer chromatography, while the expression of GD3 gangliosides was estimated by flow cytometry with a monoclonal antibody. The radiation-cell survival curves of most clones and variants derived from M4Be showed a zero dose extrapolation clearly lower than 100%, suggesting that two populations of cells of very different radiosensitivity coexist within each of these clones and variants. Although the proportion of radiosensitive cells could be estimated from the shape of the survival curve, its radiosensitivity is too high to be properly evaluated by the colony assay. The eight survival curves differ essentially in the proportion of radiosensitive cells--which varied from 0% to 40% among M4Be and its seven sublines--whereas the cellular radiosensitivity of the radioresistant population was similar among them. The metastatic potential in vivo of M4Be and its seven sublines was not significantly related to the cellular radiosensitivity of their corresponding radioresistant population, but significantly increased with the fraction of radiosensitive cells. This relationship is valid only when the highly metastatic cells are cultured for no more than five passages in vitro as the fraction of radiosensitive cells is rapidly lost during subcultures. The relationship remains valid in vivo as metastatic melanoma-bearing newborn rats whole body irradiated with 20 cGy show no lung metastasis compared with controls. The radiosensitive cell fraction is inversely correlated with both the total ganglioside content (r = 0.84, P < 0.02) and the number of cells positively labelled with the monoclonal antibody directed to GD3 (r = 0.92, P < 0.001). The incubation of a radiosensitive clone with the exogenous bovine brain ganglioside GM1 significantly increases the proportion of radioresistant cells and suppresses its metastatic potential, while the inhibition of the endogenous gangliosides synthesis in the radioresistant cell line M4Be increases the proportion of radiosensitive cells. This study provides a possible explanation for the correlation between the metastatic potential and the proportion of radiosensitive cells within the seven sublines derived from a single parental human melanoma cell line.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER P. Mouse lymphoma cells with different radiosensitivities. Nature. 1961 Nov 11;192:572–573. doi: 10.1038/192572a0. [DOI] [PubMed] [Google Scholar]
- Allalunis-Turner M. J., Barron G. M., Day R. S., 3rd, Dobler K. D., Mirzayans R. Isolation of two cell lines from a human malignant glioma specimen differing in sensitivity to radiation and chemotherapeutic drugs. Radiat Res. 1993 Jun;134(3):349–354. [PubMed] [Google Scholar]
- Bailly M., Bertrand S., Doré J. F. Human tumor spontaneous metastasis in immunosuppressed newborn rats. I. Characterization of the bioassay. Int J Cancer. 1991 Sep 30;49(3):457–466. doi: 10.1002/ijc.2910490325. [DOI] [PubMed] [Google Scholar]
- Bailly M., Bertrand S., Doré J. F. Increased spontaneous mutation rates and prevalence of karyotype abnormalities in highly metastatic human melanoma cell lines. Melanoma Res. 1993 Feb;3(1):51–61. doi: 10.1097/00008390-199304000-00008. [DOI] [PubMed] [Google Scholar]
- Bailly M., Doré J. F. Clonal drift and role of chromosome dosage in human melanoma metastatic cell lines: a statistical analysis. Anticancer Res. 1992 Jul-Aug;12(4):1163–1172. [PubMed] [Google Scholar]
- Bailly M., Doré J. F. Human tumor spontaneous metastasis in immunosuppressed newborn rats. II. Multiple selections of human melanoma metastatic clones and variants. Int J Cancer. 1991 Nov 11;49(5):750–757. doi: 10.1002/ijc.2910490520. [DOI] [PubMed] [Google Scholar]
- Buronfosse A., Thomas C. P., Ginestet C., Doré J. F. Radiosensitivity in vitro of clonogenic and non-clonogenic glioblastoma cells obtained from a human brain tumour. C R Acad Sci III. 1994 Nov;317(11):1031–1041. [PubMed] [Google Scholar]
- Courdi A., Malaise E. P. Effect of size of lymph node metastases on the radiation response: influence of misonidazole. Radiat Res. 1980 Sep;83(3):723–731. [PubMed] [Google Scholar]
- Dahlberg W. K., Little J. B., Fletcher J. A., Suit H. D., Okunieff P. Radiosensitivity in vitro of human soft tissue sarcoma cell lines and skin fibroblasts derived from the same patients. Int J Radiat Biol. 1993 Feb;63(2):191–198. doi: 10.1080/09553009314550251. [DOI] [PubMed] [Google Scholar]
- DeWyngaert J. K., Leith J. T., Peck R. A., Jr, Bliven S. F., Zeman E. M., Marino S. A., Glicksman A. S. Differential RBE values obtained for mammary adenocarcinoma tumor cell subpopulations after 14.8-MeV neutron irradiation. Radiat Res. 1981 Oct;88(1):118–131. [PubMed] [Google Scholar]
- Denekamp J., Whitmore G. F., Jeggo P. Biphasic survival curves for XRS radiosensitive cells: subpopulations or transient expression of repair competence? Int J Radiat Biol. 1989 Apr;55(4):605–617. doi: 10.1080/09553008914550651. [DOI] [PubMed] [Google Scholar]
- Fertil B., Dertinger H., Courdi A., Malaise E. P. Mean inactivation dose: a useful concept for intercomparison of human cell survival curves. Radiat Res. 1984 Jul;99(1):73–84. [PubMed] [Google Scholar]
- Fidler I. J. Selection of successive tumour lines for metastasis. Nat New Biol. 1973 Apr 4;242(118):148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- Fletcher G. H. Clinical dose-response curves of human malignant epithelial tumours. Br J Radiol. 1973 Jan;46(541):1–12. doi: 10.1259/0007-1285-46-541-1. [DOI] [PubMed] [Google Scholar]
- Fu K. K., Phillips T. L., Wharam M. D. Radiation response of artificial pulmonary metastases of the EMT6 tumor. Int J Radiat Oncol Biol Phys. 1976 Jan-Feb;1(3-4):257–260. doi: 10.1016/0360-3016(76)90047-x. [DOI] [PubMed] [Google Scholar]
- Grdina D. J., Basic I., Mason K. A., Withers H. R. Radiation response of clonogenic cell populations separated from fibrosarcoma. Radiat Res. 1975 Sep;63(3):483–493. [PubMed] [Google Scholar]
- Haimovitz-Friedman A., Kan C. C., Ehleiter D., Persaud R. S., McLoughlin M., Fuks Z., Kolesnick R. N. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994 Aug 1;180(2):525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall E. J. Radiation dose-rate: a factor of importance in radiobiology and radiotherapy. Br J Radiol. 1972 Feb;45(530):81–97. doi: 10.1259/0007-1285-45-530-81. [DOI] [PubMed] [Google Scholar]
- Hill H. Z., Hill G. J., Miller C. F., Kwong F., Purdy J. Radiation and melanoma: response of B16 mouse tumor cells and clonal lines to in vitro irradiation. Radiat Res. 1979 Nov;80(2):259–276. [PubMed] [Google Scholar]
- Hosoi Y., Sakamoto K. Suppressive effect of low dose total body irradiation on lung metastasis: dose dependency and effective period. Radiother Oncol. 1993 Feb;26(2):177–179. doi: 10.1016/0167-8140(93)90101-d. [DOI] [PubMed] [Google Scholar]
- Jenkins V. K., Barranco S. C., Townsend C. M., Jr, Perry R. R., Ives K. L. Differential response to gamma radiation of human stomach cancer cells in vitro. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Aug;50(2):269–278. doi: 10.1080/09553008614550651. [DOI] [PubMed] [Google Scholar]
- Kono K., Tsuchida T., Kern D. H., Irie R. Ganglioside composition of human melanoma and response to antitumor treatment. Cancer Invest. 1990;8(2):161–167. doi: 10.3109/07357909009017561. [DOI] [PubMed] [Google Scholar]
- Leith J. T., Dexter D. L., DeWyngaert J. K., Zeman E. M., Chu M. Y., Calabresi P., Glicksman A. S. Differential responses to x-irradiation of subpopulations of two heterogeneous human carcinomas in vitro. Cancer Res. 1982 Jul;42(7):2556–2561. [PubMed] [Google Scholar]
- Li R., Villacreses N., Ladisch S. Human tumor gangliosides inhibit murine immune responses in vivo. Cancer Res. 1995 Jan 15;55(2):211–214. [PubMed] [Google Scholar]
- Liotta L. A., Kohn E. Cancer invasion and metastases. JAMA. 1990 Feb 23;263(8):1123–1126. [PubMed] [Google Scholar]
- Loeffler J. S., Harris J. R., Dahlberg W. K., Little J. B. In vitro radiosensitivity of human diploid fibroblasts derived from women with unusually sensitive clinical responses to definitive radiation therapy for breast cancer. Radiat Res. 1990 Feb;121(2):227–231. [PubMed] [Google Scholar]
- Mothersill C., Harney J., Lyng F., Cottell D., Parsons K., Murphy D. M., Seymour C. B. Primary explants of human uroepithelium show an unusual response to low-dose irradiation with cobalt-60 gamma rays. Radiat Res. 1995 May;142(2):181–187. [PubMed] [Google Scholar]
- Portoukalian J., Bouchon B. Hydrolysis of all gangliosides, including GM1 and GM2, on thin-layer plates by Vibrio cholerae neuraminidase. J Chromatogr. 1986 Aug 2;380(2):386–392. doi: 10.1016/s0378-4347(00)83668-3. [DOI] [PubMed] [Google Scholar]
- Portoukalian J., Carrel S., Doré J. F., Rümke P. Humoral immune response in disease-free advanced melanoma patients after vaccination with melanoma-associated gangliosides. EORTC Cooperative Melanoma Group. Int J Cancer. 1991 Dec 2;49(6):893–899. doi: 10.1002/ijc.2910490616. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan N., McClain D. E., Catravas G. N. Membranes as sensitive targets in thymocyte apoptosis. Int J Radiat Biol. 1993 Jun;63(6):693–701. doi: 10.1080/09553009314552091. [DOI] [PubMed] [Google Scholar]
- Rantanen V., Grénman S., Kulmala J., Jaakkola M., Lakkala T., Sajantila A., Klemi P., Grénman R. Characterization and radiosensitivity of UT-EC-2A and UT-EC-2B, two new highly radiosensitive endometrial cancer cell lines derived from a primary and metastatic tumor of the same patient. Gynecol Oncol. 1995 Jan;56(1):53–62. doi: 10.1006/gyno.1995.1009. [DOI] [PubMed] [Google Scholar]
- Rofstad E. K. Radiation sensitivity in vitro of primary tumors and metastatic lesions of malignant melanoma. Cancer Res. 1992 Aug 15;52(16):4453–4457. [PubMed] [Google Scholar]
- Suit H., Allam A., Allalunis-Turner J., Brock W., Girinsky T., Hill S., Hunter N., Milas L., Pearcey R., Peters L. Is tumor cell radiation resistance correlated with metastatic ability? Cancer Res. 1994 Apr 1;54(7):1736–1741. [PubMed] [Google Scholar]
- Thomas C. P., Buronfosse A., Combaret V., Pedron S., Fertil B., Portoukalian J. Gangliosides protect human melanoma cells from ionizing radiation-induced clonogenic cell death. Glycoconj J. 1996 Jun;13(3):377–384. doi: 10.1007/BF00731470. [DOI] [PubMed] [Google Scholar]
- Thomas C. P., Buronfosse A., Fertil B., Portoukalian J. Surface expression of GD3 disialogangliosides in human melanoma cells is correlated to both metastatic potential in vivo and radiosensitivity in vitro. C R Acad Sci III. 1995 Dec;318(12):1233–1238. [PubMed] [Google Scholar]
- Thomas C. P., Buronfosse A., Portoukalian J., Fertil B. Correlation between the radiosensitivity in vitro of clones and variants derived from a human melanoma cell line and their spontaneous metastatic potential in vivo. Cancer Lett. 1995 Jan 27;88(2):221–225. doi: 10.1016/0304-3835(94)03624-r. [DOI] [PubMed] [Google Scholar]
- Uzawa A., Suzuki G., Nakata Y., Akashi M., Ohyama H., Akanuma A. Radiosensitivity of CD45RO+ memory and CD45RO- naive T cells in culture. Radiat Res. 1994 Jan;137(1):25–33. [PubMed] [Google Scholar]
- Welch D. R., Milas L., Tomasovic S. P., Nicolson G. L. Heterogeneous response and clonal drift of sensitivities of metastatic 13762NF mammary adenocarcinoma clones to gamma-radiation in vitro. Cancer Res. 1983 Jan;43(1):6–10. [PubMed] [Google Scholar]
- Yang X., Darling J. L., McMillan T. J., Peacock J. H., Steel G. G. Heterogeneity of radiosensitivity in a human glioma cell line. Int J Radiat Oncol Biol Phys. 1992;22(1):103–108. doi: 10.1016/0360-3016(92)90988-t. [DOI] [PubMed] [Google Scholar]
- Zebda N., Bailly M., Brown S., Doré J. F., Berthier-Vergnes O. Expression of PNA-binding sites on specific glycoproteins by human melanoma cells is associated with a high metastatic potential. J Cell Biochem. 1994 Feb;54(2):161–173. doi: 10.1002/jcb.240540205. [DOI] [PubMed] [Google Scholar]
- Zebda N., Pedron S., Rebbaa A., Portoukalian J., Berthier-Vergnes O. Deficiency of ganglioside biosynthesis in metastatic human melanoma cells: relevance of CMP-NeuAc:LacCer alpha 2-3 sialyltransferase (GM3 synthase). FEBS Lett. 1995 Apr 3;362(2):161–164. doi: 10.1016/0014-5793(95)00234-z. [DOI] [PubMed] [Google Scholar]


