Abstract
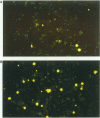
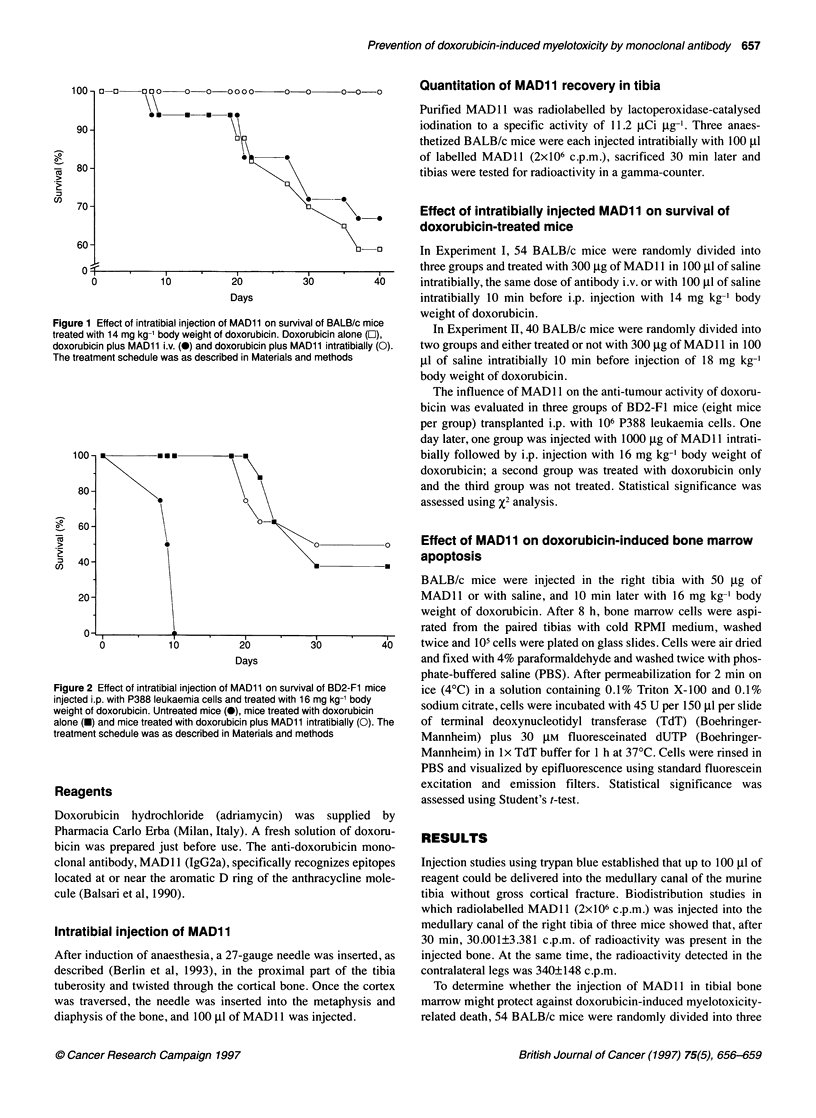
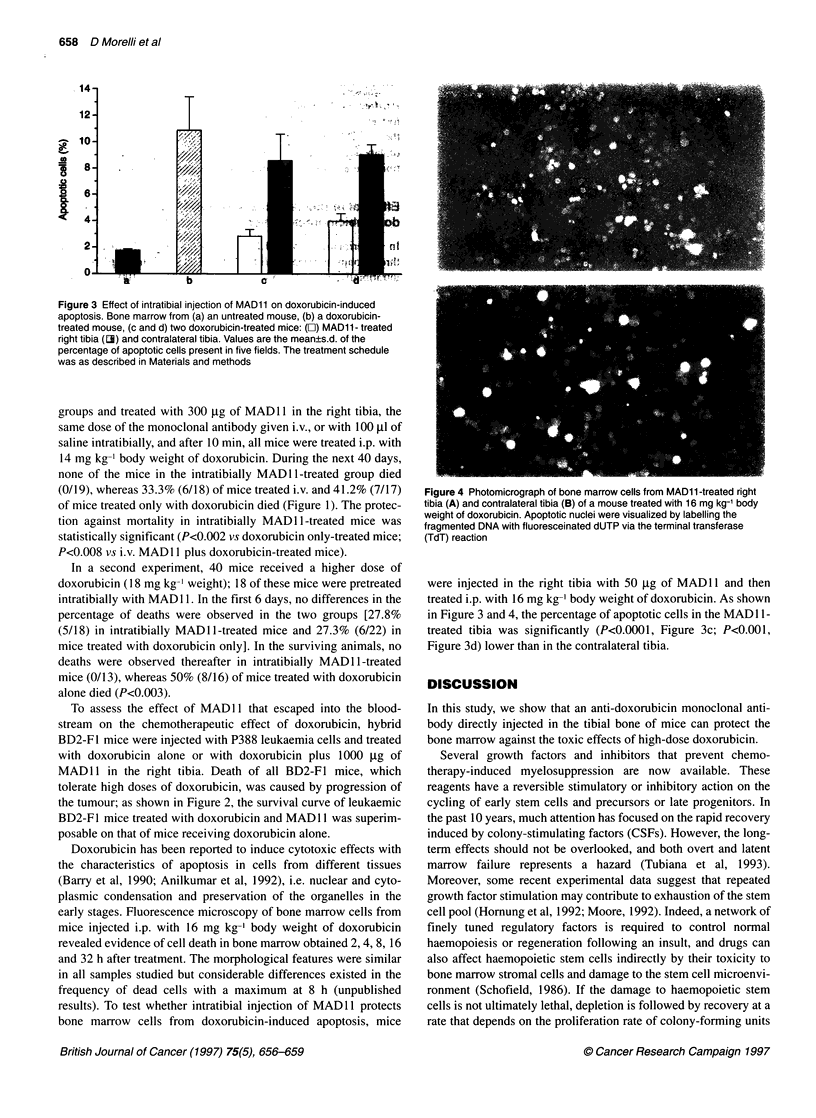
With few exceptions, the major limit to high-dose chemotherapeutic treatments is the severity and duration of drug-induced myelosuppression. We have recently developed a monoclonal antibody, MAD11, which reacts with the potent anti-tumour antibiotic doxorubicin and other anthracyclines. To protect directly pluripotent stem cells and cells of the haematopoietic microenvironment in the bone marrow against doxorubicin cytotoxicity, the monoclonal antibody MAD11 was injected into the tibial bone of mice before chemotherapeutic treatment. All mice pretreated intratibially with MAD11 and injected with 14 mg kg(-1) body weight of doxorubicin survived, whereas 41% of mice treated with doxorubicin alone died. At a higher dose of doxorubicin (18 mg kg(-1)), early mortality (first 6 days) was similar in the groups, but no deaths were observed thereafter in the intratibially MAD11-treated group, whereas most of the mice treated with doxorubicin alone died. Data obtained in mice injected with P388 leukaemia cells showed that the intratibial injection of MAD11 did not compromise the anti-tumoral activity of doxorubicin. Moreover, the administration of the anti-doxorubicin monoclonal antibody before chemotherapeutic treatment effectively reduced apoptosis induced by doxorubicin in the bone marrow cells. These data suggest the usefulness of monoclonal antibodies against chemotherapeutic drugs in the local protection of bone marrow without influencing the anti-tumour properties of the drug.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anilkumar T. V., Sarraf C. E., Hunt T., Alison M. R. The nature of cytotoxic drug-induced cell death in murine intestinal crypts. Br J Cancer. 1992 Apr;65(4):552–558. doi: 10.1038/bjc.1992.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsari A. L., Morelli D., Ménard S., Veronesi U., Colnaghi M. I. Protection against doxorubicin-induced alopecia in rats by liposome-entrapped monoclonal antibodies. FASEB J. 1994 Feb;8(2):226–230. doi: 10.1096/fasebj.8.2.8119493. [DOI] [PubMed] [Google Scholar]
- Balsari A., Morelli D., Menard S., Tagliabue E., Colnaghi M. I., Ghione M. A new monoclonal antibody recognizing anthracyclinic molecule. Anticancer Res. 1990 Jan-Feb;10(1):129–132. [PubMed] [Google Scholar]
- Balsari A., Ménard S., Colnaghi M. I., Ghione M. Anti-drug monoclonal antibodies antagonize toxic effect more than anti-tumor activity of doxorubicin. Int J Cancer. 1991 Apr 1;47(6):889–892. doi: 10.1002/ijc.2910470617. [DOI] [PubMed] [Google Scholar]
- Barry M. A., Behnke C. A., Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem Pharmacol. 1990 Nov 15;40(10):2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- Berlin O., Samid D., Donthineni-Rao R., Akeson W., Amiel D., Woods V. L., Jr Development of a novel spontaneous metastasis model of human osteosarcoma transplanted orthotopically into bone of athymic mice. Cancer Res. 1993 Oct 15;53(20):4890–4895. [PubMed] [Google Scholar]
- Cheson B. D., Lacerna L., Leyland-Jones B., Sarosy G., Wittes R. E. Autologous bone marrow transplantation. Current status and future directions. Ann Intern Med. 1989 Jan 1;110(1):51–65. doi: 10.7326/0003-4819-110-1-51. [DOI] [PubMed] [Google Scholar]
- Dexter T. M. Regulation of hemopoietic cell growth and development: experimental and clinical studies. Leukemia. 1989 Jul;3(7):469–474. [PubMed] [Google Scholar]
- Eaves C. J., Cashman J. D., Kay R. J., Dougherty G. J., Otsuka T., Gaboury L. A., Hogge D. E., Lansdorp P. M., Eaves A. C., Humphries R. K. Mechanisms that regulate the cell cycle status of very primitive hematopoietic cells in long-term human marrow cultures. II. Analysis of positive and negative regulators produced by stromal cells within the adherent layer. Blood. 1991 Jul 1;78(1):110–117. [PubMed] [Google Scholar]
- Grzegorzewski K., Ruscetti F. W., Usui N., Damia G., Longo D. L., Carlino J. A., Keller J. R., Wiltrout R. H. Recombinant transforming growth factor beta 1 and beta 2 protect mice from acutely lethal doses of 5-fluorouracil and doxorubicin. J Exp Med. 1994 Sep 1;180(3):1047–1057. doi: 10.1084/jem.180.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guigon M., Lemoine F., Najman A. Bone marrow protection. Bone Marrow Transplant. 1994 Jan;13(1):93–95. [PubMed] [Google Scholar]
- Gulati S. C., Yahalom J., Portlock C. Autologous bone marrow transplantation. Curr Probl Cancer. 1991 Jan-Feb;15(1):1–57. [PubMed] [Google Scholar]
- Henon P. R., Liang H., Beck-Wirth G., Eisenmann J. C., Lepers M., Wunder E., Kandel G. Comparison of hematopoietic and immune recovery after autologous bone marrow or blood stem cell transplants. Bone Marrow Transplant. 1992 Apr;9(4):285–291. [PubMed] [Google Scholar]
- Hornung R. L., Longo D. L. Hematopoietic stem cell depletion by restorative growth factor regimens during repeated high-dose cyclophosphamide therapy. Blood. 1992 Jul 1;80(1):77–83. [PubMed] [Google Scholar]
- Hénon P. R. Peripheral blood stem cell transplantations: past, present and future. Stem Cells. 1993 May;11(3):154–172. doi: 10.1002/stem.5530110302. [DOI] [PubMed] [Google Scholar]
- Lieschke G. J., Burgess A. W. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (1). N Engl J Med. 1992 Jul 2;327(1):28–35. doi: 10.1056/NEJM199207023270106. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The colony stimulating factors. Discovery, development, and clinical applications. Cancer. 1990 May 15;65(10):2185–2195. doi: 10.1002/1097-0142(19900515)65:10<2185::aid-cncr2820651005>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Moore M. A. Does stem cell exhaustion result from combining hematopoietic growth factors with chemotherapy? If so, how do we prevent it? Blood. 1992 Jul 1;80(1):3–7. [PubMed] [Google Scholar]
- Morelli D., Ménard S., Colnaghi M. I., Balsari A. Oral administration of anti-doxorubicin monoclonal antibody prevents chemotherapy-induced gastrointestinal toxicity in mice. Cancer Res. 1996 May 1;56(9):2082–2085. [PubMed] [Google Scholar]
- Sardini A., Villa E., Morelli D., Ghione M., Ménard S., Colnaghi M. I., Balsari A. An anti-doxorubicin monoclonal antibody modulates kinetic and dynamic characteristics of the drug. Int J Cancer. 1992 Feb 20;50(4):617–620. doi: 10.1002/ijc.2910500422. [DOI] [PubMed] [Google Scholar]
- Schofield R. Assessment of cytotoxic injury to bone marrow. Br J Cancer Suppl. 1986;7:115–125. [PMC free article] [PubMed] [Google Scholar]
- To L. B., Roberts M. M., Haylock D. N., Dyson P. G., Branford A. L., Thorp D., Ho J. Q., Dart G. W., Horvath N., Davy M. L. Comparison of haematological recovery times and supportive care requirements of autologous recovery phase peripheral blood stem cell transplants, autologous bone marrow transplants and allogeneic bone marrow transplants. Bone Marrow Transplant. 1992 Apr;9(4):277–284. [PubMed] [Google Scholar]
- Tubiana M., Carde P., Frindel E. Ways of minimising hematopoietic damage induced by radiation and cytostatic drugs--the possible role of inhibitors. Radiother Oncol. 1993 Oct;29(1):1–17. doi: 10.1016/0167-8140(93)90167-7. [DOI] [PubMed] [Google Scholar]
- Vassort F., Frindel E., Tubiana M. Effects of hydroxyurea on the kinetics of colony forming units of bone marrow in the mouse. Cell Tissue Kinet. 1971 Sep;4(5):423–431. doi: 10.1111/j.1365-2184.1971.tb01551.x. [DOI] [PubMed] [Google Scholar]
- Weiss R. B. The anthracyclines: will we ever find a better doxorubicin? Semin Oncol. 1992 Dec;19(6):670–686. [PubMed] [Google Scholar]