Abstract
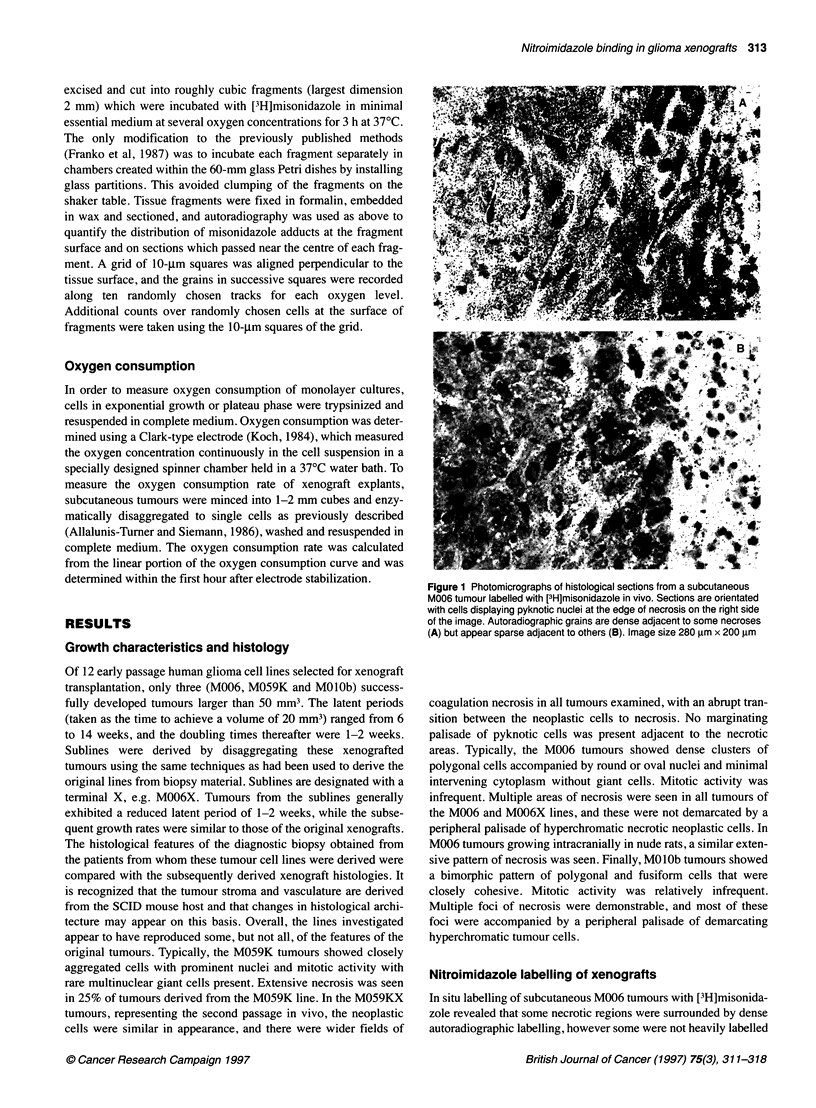
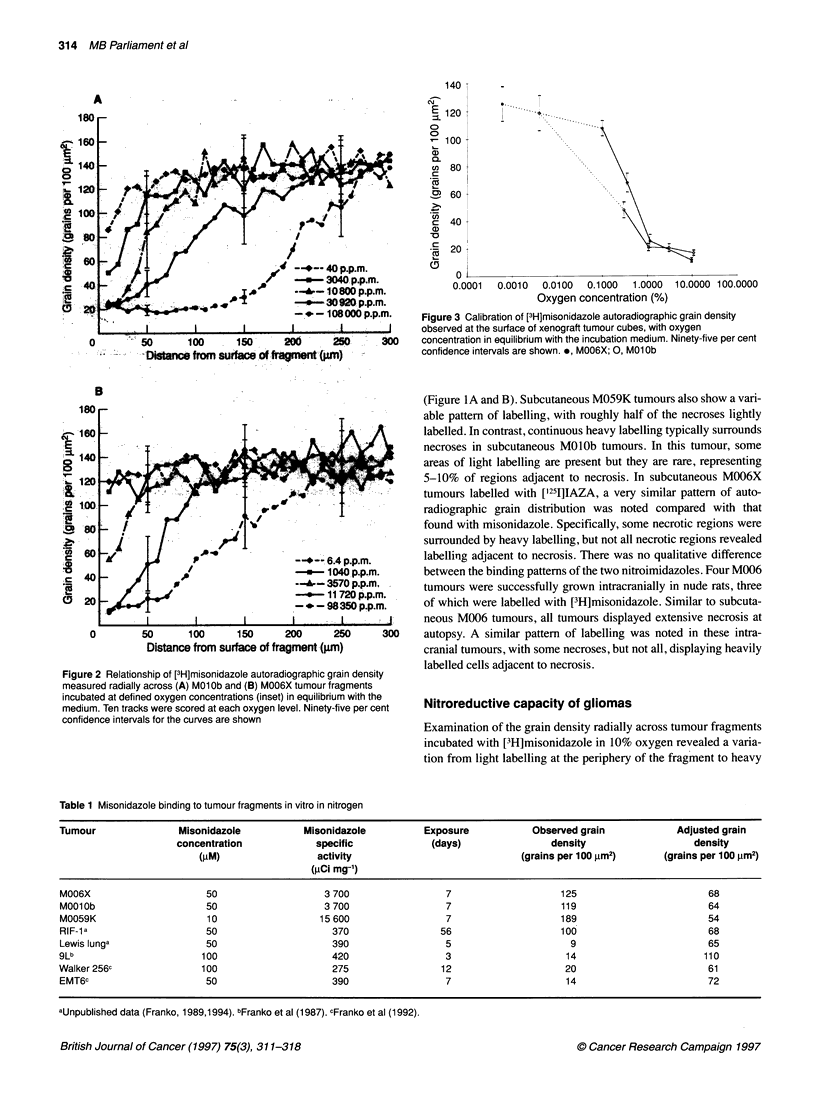
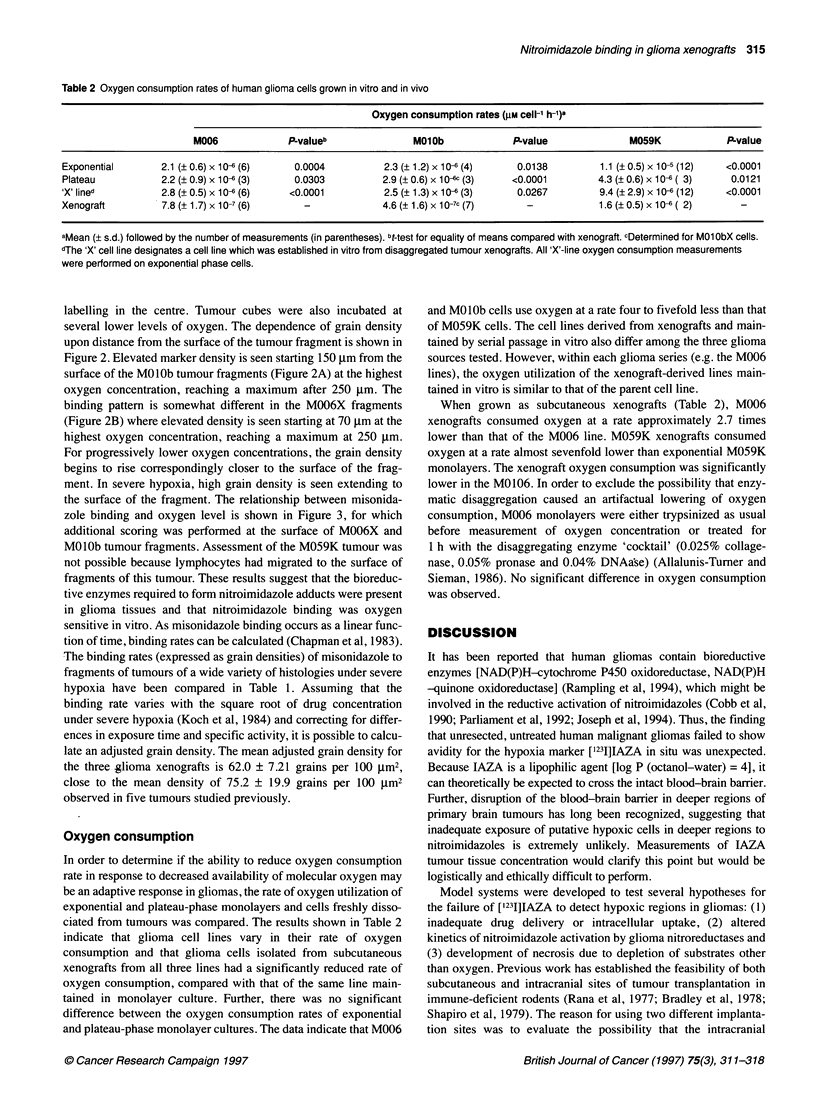
In contrast to reports of extensive hypoxia in human gliomas in situ measured by pO2 histography, non-invasive methods of assessing glioma oxygenation, including nitroimidazole binding, have yielded surprisingly contradictory results. In order to investigate the relationship of necrosis, hypoxia, nitroreductase activity and cellular respiration in human gliomas, subcutaneous models using the human glioma cell lines M059K, M006 and M010b were developed in the murine SCID host. Intracranial growth of the M006 line was achieved in nude rats. The nitroreductive capacity of glioma cell lines was assessed and found to be similar to transplanted tumours previously reported in the literature. This suggests that if substantial numbers of viable hypoxic cells were present in situ in gliomas, then nitroimidazole-binding techniques should be capable of identifying them. Inter-tumour variability in the amount of necrosis was seen. M006 xenografts growing in subcutaneous and intracranial sites revealed extensive necrotic regions histologically, some of which were surrounded by cells labelled heavily for [3H]misonidazole, while other areas were lightly labelled. Similar binding patterns were seen for subcutaneous M059K tumours, while subcutaneous M010b tumours display necroses of which almost all were surrounded by heavily labelled cells. The oxygen consumption rates of tumour cell lines grown in vivo, in which venous pO2 concentrations are of the order of 2-5%, were two to sevenfold less than those of the same lines grown as monolayers in vitro under oxygen concentrations of 18%. We postulate that glioma cell lines behave as 'oxygen conformers', in that their rate of oxygen consumption appears to vary with the availability of oxygen. Together with the misonidazole-binding data, the results in this glioma tumour model are consistent with coordinate inhibition or down-regulation of respiration under moderate hypoxia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allalunis-Turner M. J., Day R. S., 3rd, McKean J. D., Petruk K. C., Allen P. B., Aronyk K. E., Weir B. K., Huyser-Wierenga D., Fulton D. S., Urtasun R. C. Glutathione levels and chemosensitizing effects of buthionine sulfoximine in human malignant glioma cells. J Neurooncol. 1991 Oct;11(2):157–164. doi: 10.1007/BF02390175. [DOI] [PubMed] [Google Scholar]
- Allalunis-Turner M. J., Siemann D. W. Recovery of cell subpopulations from human tumour xenografts following dissociation with different enzymes. Br J Cancer. 1986 Oct;54(4):615–622. doi: 10.1038/bjc.1986.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleehen N. M., Wiltshire C. R., Plowman P. N., Watson J. V., Gleave J. R., Holmes A. E., Lewin W. S., Treip C. S., Hawkins T. D. A randomized study of misonidazole and radiotherapy for grade 3 and 4 cerebral astrocytoma. Br J Cancer. 1981 Apr;43(4):436–442. doi: 10.1038/bjc.1981.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley N. J., Bloom H. J., Davies A. J., Swift S. M. Growth of human gliomas in immune-deficient mice: a possible model for pre-clinical therapy studies. Br J Cancer. 1978 Aug;38(2):263–272. doi: 10.1038/bjc.1978.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard D. E., Schold S. C., Jr, Bigner S. H., Bigner D. D. Growth and chemotherapeutic response in athymic mice of tumors arising from human glioma-derived cell lines. J Neuropathol Exp Neurol. 1981 Jul;40(4):410–427. doi: 10.1097/00005072-198107000-00005. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Nilsson K., Westermark B., Pontén J., Sundström C., Larsson E., Bergh J., Påhlman S., Busch C., Collins V. P. Formation and growth of multicellular spheroids of human origin. Int J Cancer. 1983 May 15;31(5):523–533. doi: 10.1002/ijc.2910310502. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Stålnacke C. G., Acker H., Haji-Karim M., Nilsson S., Larsson B. The influence of oxygen on viability and proliferation in cellular spheroids. Int J Radiat Oncol Biol Phys. 1979 Nov-Dec;5(11-12):2011–2020. doi: 10.1016/0360-3016(79)90953-2. [DOI] [PubMed] [Google Scholar]
- Chapman J. D., Baer K., Lee J. Characteristics of the metabolism-induced binding of misonidazole to hypoxic mammalian cells. Cancer Res. 1983 Apr;43(4):1523–1528. [PubMed] [Google Scholar]
- Chapman J. D., Franko A. J., Sharplin J. A marker for hypoxic cells in tumours with potential clinical applicability. Br J Cancer. 1981 Apr;43(4):546–550. doi: 10.1038/bjc.1981.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb L. M., Hacker T., Nolan J. NAD(P)H nitroblue tetrazolium reductase levels in apparently normoxic tissues: a histochemical study correlating enzyme activity with binding of radiolabelled misonidazole. Br J Cancer. 1990 Apr;61(4):524–529. doi: 10.1038/bjc.1990.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franko A. J., Koch C. J., Boisvert D. P. Distribution of misonidazole adducts in 9L gliosarcoma tumors and spheroids: implications for oxygen distribution. Cancer Res. 1992 Jul 15;52(14):3831–3837. [PubMed] [Google Scholar]
- Franko A. J., Koch C. J., Garrecht B. M., Sharplin J., Hughes D. Oxygen dependence of binding of misonidazole to rodent and human tumors in vitro. Cancer Res. 1987 Oct 15;47(20):5367–5376. [PubMed] [Google Scholar]
- Freyer J. P., Sutherland R. M. A reduction in the in situ rates of oxygen and glucose consumption of cells in EMT6/Ro spheroids during growth. J Cell Physiol. 1985 Sep;124(3):516–524. doi: 10.1002/jcp.1041240323. [DOI] [PubMed] [Google Scholar]
- Freyer J. P., Tustanoff E., Franko A. J., Sutherland R. M. In situ oxygen consumption rates of cells in V-79 multicellular spheroids during growth. J Cell Physiol. 1984 Jan;118(1):53–61. doi: 10.1002/jcp.1041180111. [DOI] [PubMed] [Google Scholar]
- Groshar D., McEwan A. J., Parliament M. B., Urtasun R. C., Golberg L. E., Hoskinson M., Mercer J. R., Mannan R. H., Wiebe L. I., Chapman J. D. Imaging tumor hypoxia and tumor perfusion. J Nucl Med. 1993 Jun;34(6):885–888. [PubMed] [Google Scholar]
- Horten B. C., Basler G. A., Shapiro W. R. Xenograft of human malignant glial tumors into brains of nude mice. A histopatholgical study. J Neuropathol Exp Neurol. 1981 Sep;40(5):493–511. doi: 10.1097/00005072-198109000-00002. [DOI] [PubMed] [Google Scholar]
- Ito M., Lammertsma A. A., Wise R. J., Bernardi S., Frackowiak R. S., Heather J. D., McKenzie C. G., Thomas D. G., Jones T. Measurement of regional cerebral blood flow and oxygen utilisation in patients with cerebral tumours using 15O and positron emission tomography: analytical techniques and preliminary results. Neuroradiology. 1982;23(2):63–74. doi: 10.1007/BF00367239. [DOI] [PubMed] [Google Scholar]
- Jones T. R., Bigner S. H., Schold S. C., Jr, Eng L. F., Bigner D. D. Anaplastic human gliomas grown in athymic mice. Morphology and glial fibrillary acidic protein expression. Am J Pathol. 1981 Dec;105(3):316–327. [PMC free article] [PubMed] [Google Scholar]
- Joseph P., Jaiswal A. K., Stobbe C. C., Chapman J. D. The role of specific reductases in the intracellular activation and binding of 2-nitroimidazoles. Int J Radiat Oncol Biol Phys. 1994 May 15;29(2):351–355. doi: 10.1016/0360-3016(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Koch C. J. A thin-film culturing technique allowing rapid gas-liquid equilibration (6 sec) with no toxicity to mammalian cells. Radiat Res. 1984 Feb;97(2):434–442. [PubMed] [Google Scholar]
- Kornblith P. L., Cummins C. J., Smith B. H., Brooks R. A., Patronas N. J., Di Chiro G. Correlation of experimental and clinical studies of metabolism by PET scanning. Prog Exp Tumor Res. 1984;27:170–178. doi: 10.1159/000408229. [DOI] [PubMed] [Google Scholar]
- Lammertsma A. A., Wise R. J., Cox T. C., Thomas D. G., Jones T. Measurement of blood flow, oxygen utilisation, oxygen extraction ratio, and fractional blood volume in human brain tumours and surrounding oedematous tissue. Br J Radiol. 1985 Aug;58(692):725–734. doi: 10.1259/0007-1285-58-692-725. [DOI] [PubMed] [Google Scholar]
- Moulder J. E., Rockwell S. Hypoxic fractions of solid tumors: experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys. 1984 May;10(5):695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- Nelson J. S., Tsukada Y., Schoenfeld D., Fulling K., Lamarche J., Peress N. Necrosis as a prognostic criterion in malignant supratentorial, astrocytic gliomas. Cancer. 1983 Aug 1;52(3):550–554. doi: 10.1002/1097-0142(19830801)52:3<550::aid-cncr2820520327>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Olive P. L., Vikse C., Trotter M. J. Measurement of oxygen diffusion distance in tumor cubes using a fluorescent hypoxia probe. Int J Radiat Oncol Biol Phys. 1992;22(3):397–402. doi: 10.1016/0360-3016(92)90840-e. [DOI] [PubMed] [Google Scholar]
- Parliament M. B., Wiebe L. I., Franko A. J. Nitroimidazole adducts as markers for tissue hypoxia: mechanistic studies in aerobic normal tissues and tumour cells. Br J Cancer. 1992 Dec;66(6):1103–1108. doi: 10.1038/bjc.1992.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampling R., Cruickshank G., Lewis A. D., Fitzsimmons S. A., Workman P. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int J Radiat Oncol Biol Phys. 1994 Jun 15;29(3):427–431. doi: 10.1016/0360-3016(94)90432-4. [DOI] [PubMed] [Google Scholar]
- Rana M. W., Pinkerton H., Thornton H., Nagy D. Heterotransplantation of human glioblastoma multiforme and meningioma to nude mice. Proc Soc Exp Biol Med. 1977 May;155(1):85–88. doi: 10.3181/00379727-155-39750. [DOI] [PubMed] [Google Scholar]
- Rockwell S., Moulder J. E. Hypoxic fractions of human tumors xenografted into mice: a review. Int J Radiat Oncol Biol Phys. 1990 Jul;19(1):197–202. doi: 10.1016/0360-3016(90)90154-c. [DOI] [PubMed] [Google Scholar]
- Shapiro W. R., Basler G. A., Chernik N. L., Posner J. B. Human brain tumor transplantation into nude mice. J Natl Cancer Inst. 1979 Mar;62(3):447–453. doi: 10.1093/jnci/62.3.447. [DOI] [PubMed] [Google Scholar]
- Urtasun R. C., Koch C. J., Franko A. J., Raleigh J. A., Chapman J. D. A novel technique for measuring human tissue pO2 at the cellular level. Br J Cancer. 1986 Sep;54(3):453–457. doi: 10.1038/bjc.1986.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urtasun R., Band P., Chapman J. D., Feldstein M. L., Mielke B., Fryer C. Radiation and high-dose metronidazole in supratentorial glioblastomas. N Engl J Med. 1976 Jun 17;294(25):1364–1367. doi: 10.1056/NEJM197606172942503. [DOI] [PubMed] [Google Scholar]
- Valk P. E., Mathis C. A., Prados M. D., Gilbert J. C., Budinger T. F. Hypoxia in human gliomas: demonstration by PET with fluorine-18-fluoromisonidazole. J Nucl Med. 1992 Dec;33(12):2133–2137. [PubMed] [Google Scholar]
- WARBURG O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Wise R. J., Thomas D. G., Lammertsma A. A., Rhodes C. G. PET scanning of human brain tumors. Prog Exp Tumor Res. 1984;27:154–169. doi: 10.1159/000408228. [DOI] [PubMed] [Google Scholar]



