Abstract
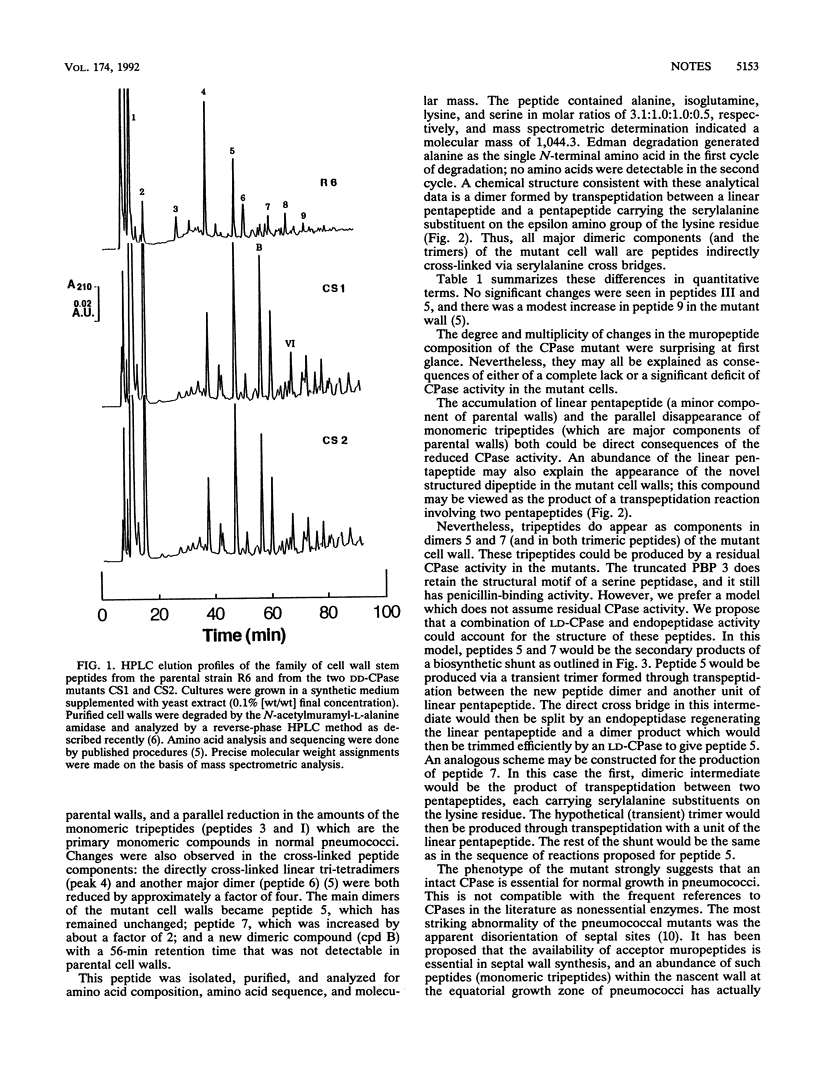
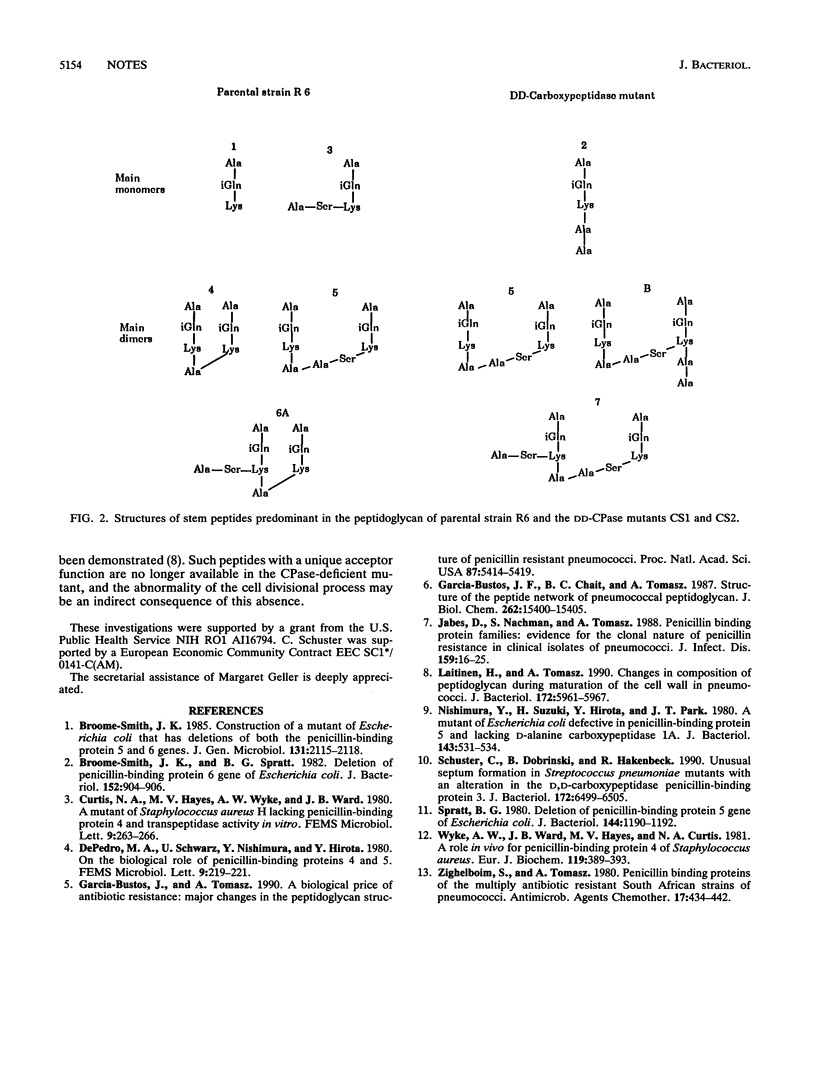
The muropeptide composition of a Streptococcus pneumoniae mutant in which the DD-carboxypeptidase (penicillin-binding protein 3) gene was interrupted by plasmid insertion close to the 3' end of the gene was examined. Extensive compositional changes were observed: the linear pentapeptide, a minor component of the parental cells, became the most abundant monomeric peptide in the mutant wall, while the proportion of tripeptides that represent the main monomers in the parental cells was greatly reduced. The amount of the major dimer of parental cells, the directly cross-linked tri-tetrapeptide, was also reduced by a factor of 4. It was partially replaced by a novel dimer: the cross-linked product of a linear pentapeptide and a pentapeptide carrying a serylalanine dipeptide substituent on the epsilon-NH2 group of its lysine residue. This dimer together with two other dimeric peptides, each containing the serylalanine cross bridge, became the quantitatively major components of the mutant peptidoglycan.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broome-Smith J. K. Construction of a mutant of Escherichia coli that has deletions of both the penicillin-binding protein 5 and 6 genes. J Gen Microbiol. 1985 Aug;131(8):2115–2118. doi: 10.1099/00221287-131-8-2115. [DOI] [PubMed] [Google Scholar]
- Broome-Smith J. K., Spratt B. G. Deletion of the penicillin-binding protein 6 gene of Escherichia coli. J Bacteriol. 1982 Nov;152(2):904–906. doi: 10.1128/jb.152.2.904-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bustos J. F., Chait B. T., Tomasz A. Structure of the peptide network of pneumococcal peptidoglycan. J Biol Chem. 1987 Nov 15;262(32):15400–15405. [PubMed] [Google Scholar]
- Garcia-Bustos J., Tomasz A. A biological price of antibiotic resistance: major changes in the peptidoglycan structure of penicillin-resistant pneumococci. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5415–5419. doi: 10.1073/pnas.87.14.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabes D., Nachman S., Tomasz A. Penicillin-binding protein families: evidence for the clonal nature of penicillin resistance in clinical isolates of pneumococci. J Infect Dis. 1989 Jan;159(1):16–25. doi: 10.1093/infdis/159.1.16. [DOI] [PubMed] [Google Scholar]
- Laitinen H., Tomasz A. Changes in composition of peptidoglycan during maturation of the cell wall in pneumococci. J Bacteriol. 1990 Oct;172(10):5961–5967. doi: 10.1128/jb.172.10.5961-5967.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Suzuki H., Hirota Y., Park J. T. A mutant of Escherichia coli defective in penicillin-binding protein 5 and lacking D-alanine carboxypeptidase IA. J Bacteriol. 1980 Jul;143(1):531–534. doi: 10.1128/jb.143.1.531-534.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster C., Dobrinski B., Hakenbeck R. Unusual septum formation in Streptococcus pneumoniae mutants with an alteration in the D,D-carboxypeptidase penicillin-binding protein 3. J Bacteriol. 1990 Nov;172(11):6499–6505. doi: 10.1128/jb.172.11.6499-6505.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Deletion of the penicillin-binding protein 5 gene of Escherichia coli. J Bacteriol. 1980 Dec;144(3):1190–1192. doi: 10.1128/jb.144.3.1190-1192.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke A. W., Ward J. B., Hayes M. V., Curtis N. A. A role in vivo for penicillin-binding protein-4 of Staphylococcus aureus. Eur J Biochem. 1981 Oct;119(2):389–393. doi: 10.1111/j.1432-1033.1981.tb05620.x. [DOI] [PubMed] [Google Scholar]
- Zighelboim S., Tomasz A. Penicillin-binding proteins of multiply antibiotic-resistant South African strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):434–442. doi: 10.1128/aac.17.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]



