Abstract
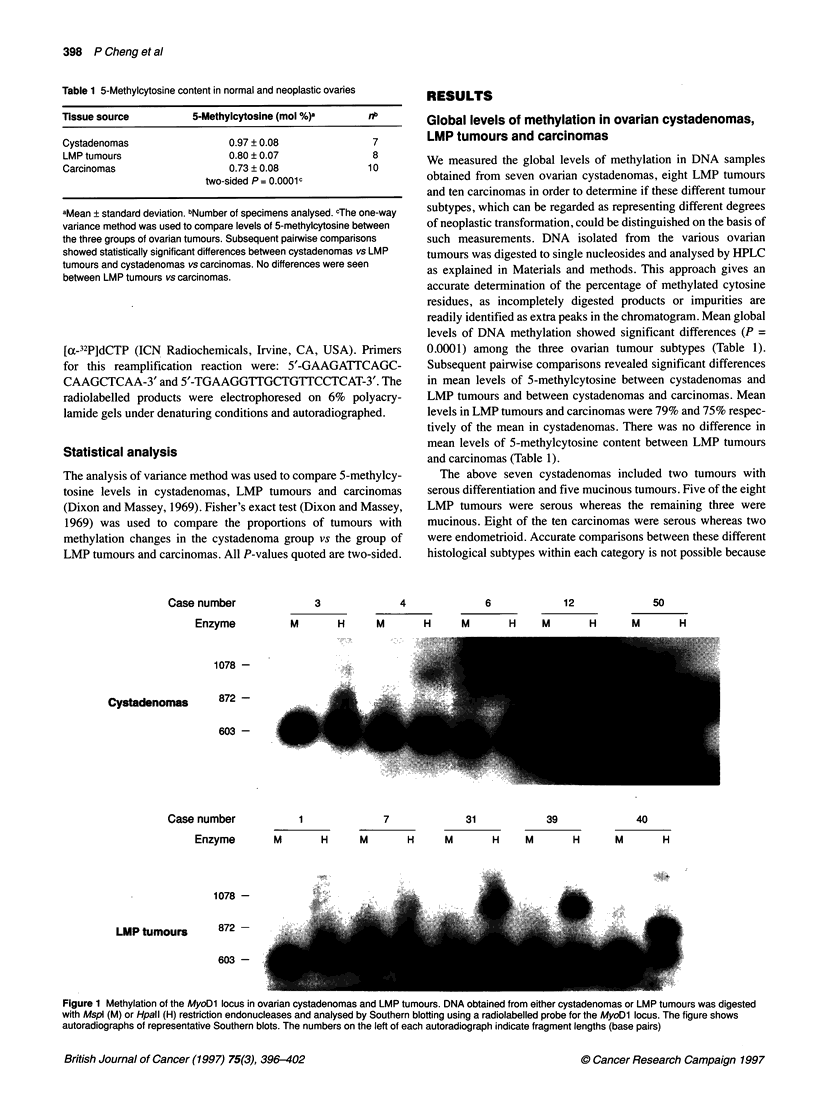
We compared global levels of DNA methylation as well as methylation of a specific locus (MyoD1) in ovarian cystadenomas, ovarian tumours of low malignant potential (LMP) and ovarian carcinomas to investigate the association between changes in DNA methylation and ovarian tumour development. As we realized that cystadenomas showed different methylation patterns from both LMP tumours and carcinomas, we verified their monoclonal origin as a means of confirming their true neoplastic nature. High-pressure liquid chromatographic (HPLC) analyses showed that global methylation levels in LMP tumours and carcinomas were 21% and 25% lower than in cystadenomas respectively (P = 0.0001 by one-way variance analysis). Changes in the methylation status of the MyoD1 locus were not seen in any of ten cystadenomas analysed but were present in five of ten LMP tumours and in five of ten carcinomas (P = 0.03). These findings suggest that alterations in DNA methylation are absent (or at least not as extensive) in ovarian cystadenomas, but are present in LMP tumours, the phenotypic features of which are intermediate between those of benign and malignant ovarian tumours. The results also emphasize the merit of distinguishing ovarian LMP tumours from cystadenomas, in spite of their similar clinical characteristics.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Zoghbi H. Y., Moseley A. B., Rosenblatt H. M., Belmont J. W. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992 Dec;51(6):1229–1239. [PMC free article] [PubMed] [Google Scholar]
- Antequera F., Boyes J., Bird A. High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell. 1990 Aug 10;62(3):503–514. doi: 10.1016/0092-8674(90)90015-7. [DOI] [PubMed] [Google Scholar]
- Balmain A. Cancer. Exploring the bowels of DNA methylation. Curr Biol. 1995 Sep 1;5(9):1013–1016. doi: 10.1016/s0960-9822(95)00204-1. [DOI] [PubMed] [Google Scholar]
- Bhave M. R., Wilson M. J., Poirier L. A. c-H-ras and c-K-ras gene hypomethylation in the livers and hepatomas of rats fed methyl-deficient, amino acid-defined diets. Carcinogenesis. 1988 Mar;9(3):343–348. doi: 10.1093/carcin/9.3.343. [DOI] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Bird A. The essentials of DNA methylation. Cell. 1992 Jul 10;70(1):5–8. doi: 10.1016/0092-8674(92)90526-i. [DOI] [PubMed] [Google Scholar]
- Braun T., Bober E., Rudnicki M. A., Jaenisch R., Arnold H. H. MyoD expression marks the onset of skeletal myogenesis in Myf-5 mutant mice. Development. 1994 Nov;120(11):3083–3092. doi: 10.1242/dev.120.11.3083. [DOI] [PubMed] [Google Scholar]
- Cheng P. C., Gosewehr J. A., Kim T. M., Velicescu M., Wan M., Zheng J., Felix J. C., Cofer K. F., Luo P., Biela B. H. Potential role of the inactivated X chromosome in ovarian epithelial tumor development. J Natl Cancer Inst. 1996 Apr 17;88(8):510–518. doi: 10.1093/jnci/88.8.510. [DOI] [PubMed] [Google Scholar]
- Ehlen T., Dubeau L. Loss of heterozygosity on chromosomal segments 3p, 6q and 11p in human ovarian carcinomas. Oncogene. 1990 Feb;5(2):219–223. [PubMed] [Google Scholar]
- Ehrlich M., Wang R. Y. 5-Methylcytosine in eukaryotic DNA. Science. 1981 Jun 19;212(4501):1350–1357. doi: 10.1126/science.6262918. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Gehrke C. W., Kuo K. C., Ehrlich M. Reduced genomic 5-methylcytosine content in human colonic neoplasia. Cancer Res. 1988 Mar 1;48(5):1159–1161. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983 Jan 6;301(5895):89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- Felgner J., Kreipe H., Heidorn K., Jaquet K., Zschunke F., Radzun H. J., Parwaresch M. R. Increased methylation of the c-fms protooncogene in acute myelomonocytic leukemias. Pathobiology. 1991;59(4):293–298. doi: 10.1159/000163666. [DOI] [PubMed] [Google Scholar]
- Gama-Sosa M. A., Slagel V. A., Trewyn R. W., Oxenhandler R., Kuo K. C., Gehrke C. W., Ehrlich M. The 5-methylcytosine content of DNA from human tumors. Nucleic Acids Res. 1983 Oct 11;11(19):6883–6894. doi: 10.1093/nar/11.19.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke C. W., McCune R. A., Gama-Sosa M. A., Ehrlich M., Kuo K. C. Quantitative reversed-phase high-performance liquid chromatography of major and modified nucleosides in DNA. J Chromatogr. 1984 Sep 28;301(1):199–219. doi: 10.1016/s0021-9673(01)89189-5. [DOI] [PubMed] [Google Scholar]
- Goelz S. E., Vogelstein B., Hamilton S. R., Feinberg A. P. Hypomethylation of DNA from benign and malignant human colon neoplasms. Science. 1985 Apr 12;228(4696):187–190. doi: 10.1126/science.2579435. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Zulueta M., Bender C. M., Yang A. S., Nguyen T., Beart R. W., Van Tornout J. M., Jones P. A. Methylation of the 5' CpG island of the p16/CDKN2 tumor suppressor gene in normal and transformed human tissues correlates with gene silencing. Cancer Res. 1995 Oct 15;55(20):4531–4535. [PubMed] [Google Scholar]
- Greger V., Passarge E., Höpping W., Messmer E., Horsthemke B. Epigenetic changes may contribute to the formation and spontaneous regression of retinoblastoma. Hum Genet. 1989 Sep;83(2):155–158. doi: 10.1007/BF00286709. [DOI] [PubMed] [Google Scholar]
- Hanada M., Delia D., Aiello A., Stadtmauer E., Reed J. C. bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood. 1993 Sep 15;82(6):1820–1828. [PubMed] [Google Scholar]
- Herman J. G., Latif F., Weng Y., Lerman M. I., Zbar B., Liu S., Samid D., Duan D. S., Gnarra J. R., Linehan W. M. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9700–9704. doi: 10.1073/pnas.91.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A., Wolkowicz M. J., Rideout W. M., 3rd, Gonzales F. A., Marziasz C. M., Coetzee G. A., Tapscott S. J. De novo methylation of the MyoD1 CpG island during the establishment of immortal cell lines. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6117–6121. doi: 10.1073/pnas.87.16.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet I., Yisraeli J., Cedar H. Effect of regional DNA methylation on gene expression. Proc Natl Acad Sci U S A. 1985 May;82(9):2560–2564. doi: 10.1073/pnas.82.9.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurman R. J., Trimble C. L. The behavior of serous tumors of low malignant potential: are they ever malignant? Int J Gynecol Pathol. 1993 Apr;12(2):120–127. doi: 10.1097/00004347-199304000-00006. [DOI] [PubMed] [Google Scholar]
- Laird P. W., Jaenisch R. DNA methylation and cancer. Hum Mol Genet. 1994;3(Spec No):1487–1495. doi: 10.1093/hmg/3.suppl_1.1487. [DOI] [PubMed] [Google Scholar]
- Li E., Beard C., Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993 Nov 25;366(6453):362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- Li E., Bestor T. H., Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992 Jun 12;69(6):915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Lipsanen V., Leinonen P., Alhonen L., Jänne J. Hypomethylation of ornithine decarboxylase gene and erb-A1 oncogene in human chronic lymphatic leukemia. Blood. 1988 Dec;72(6):2042–2044. [PubMed] [Google Scholar]
- Makos M., Nelkin B. D., Reiter R. E., Gnarra J. R., Brooks J., Isaacs W., Linehan M., Baylin S. B. Regional DNA hypermethylation at D17S5 precedes 17p structural changes in the progression of renal tumors. Cancer Res. 1993 Jun 15;53(12):2719–2722. [PubMed] [Google Scholar]
- Merlo A., Herman J. G., Mao L., Lee D. J., Gabrielson E., Burger P. C., Baylin S. B., Sidransky D. 5' CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995 Jul;1(7):686–692. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Nelkin B. D., Przepiorka D., Burke P. J., Thomas E. D., Baylin S. B. Abnormal methylation of the calcitonin gene marks progression of chronic myelogenous leukemia. Blood. 1991 Jun 1;77(11):2431–2434. [PubMed] [Google Scholar]
- Ray J. S., Harbison M. L., McClain R. M., Goodman J. I. Alterations in the methylation status and expression of the raf oncogene in phenobarbital-induced and spontaneous B6C3F1 mouse liver tumors. Mol Carcinog. 1994 Mar;9(3):155–166. doi: 10.1002/mc.2940090307. [DOI] [PubMed] [Google Scholar]
- Rideout W. M., 3rd, Eversole-Cire P., Spruck C. H., 3rd, Hustad C. M., Coetzee G. A., Gonzales F. A., Jones P. A. Progressive increases in the methylation status and heterochromatinization of the myoD CpG island during oncogenic transformation. Mol Cell Biol. 1994 Sep;14(9):6143–6152. doi: 10.1128/mcb.14.9.6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selig S., Ariel M., Goitein R., Marcus M., Cedar H. Regulation of mouse satellite DNA replication time. EMBO J. 1988 Feb;7(2):419–426. doi: 10.1002/j.1460-2075.1988.tb02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrard R. M., Royds J. A., Rogers S., Shorthouse A. J. Patterns of methylation of the c-myc gene in human colorectal cancer progression. Br J Cancer. 1992 May;65(5):667–672. doi: 10.1038/bjc.1992.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleddens H. F., Oostra B. A., Brinkmann A. O., Trapman J. Trinucleotide repeat polymorphism in the androgen receptor gene (AR). Nucleic Acids Res. 1992 Mar 25;20(6):1427–1427. doi: 10.1093/nar/20.6.1427-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertino P. M., Spillare E. A., Harris C. C., Baylin S. B. Altered chromosomal methylation patterns accompany oncogene-induced transformation of human bronchial epithelial cells. Cancer Res. 1993 Apr 1;53(7):1684–1689. [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Feinberg A. P. Use of restriction fragment length polymorphisms to determine the clonal origin of human tumors. Science. 1985 Feb 8;227(4687):642–645. doi: 10.1126/science.2982210. [DOI] [PubMed] [Google Scholar]
- Zheng J., Benedict W. F., Xu H. J., Hu S. X., Kim T. M., Velicescu M., Wan M., Cofer K. F., Dubeau L. Genetic disparity between morphologically benign cysts contiguous to ovarian carcinomas and solitary cystadenomas. J Natl Cancer Inst. 1995 Aug 2;87(15):1146–1153. doi: 10.1093/jnci/87.15.1146. [DOI] [PubMed] [Google Scholar]
- Zheng J., Wan M., Zweizig S., Velicescu M., Yu M. C., Dubeau L. Histologically benign or low-grade malignant tumors adjacent to high-grade ovarian carcinomas contain molecular characteristics of high-grade carcinomas. Cancer Res. 1993 Sep 15;53(18):4138–4142. [PubMed] [Google Scholar]