Abstract
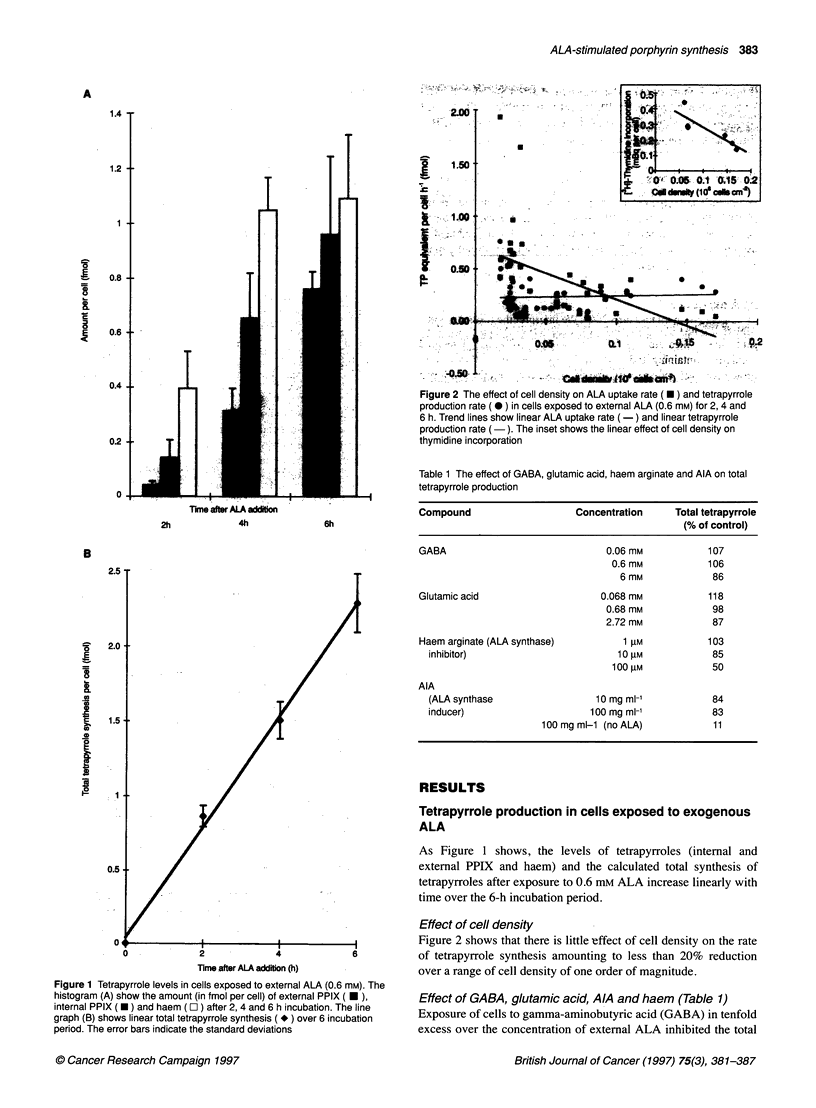
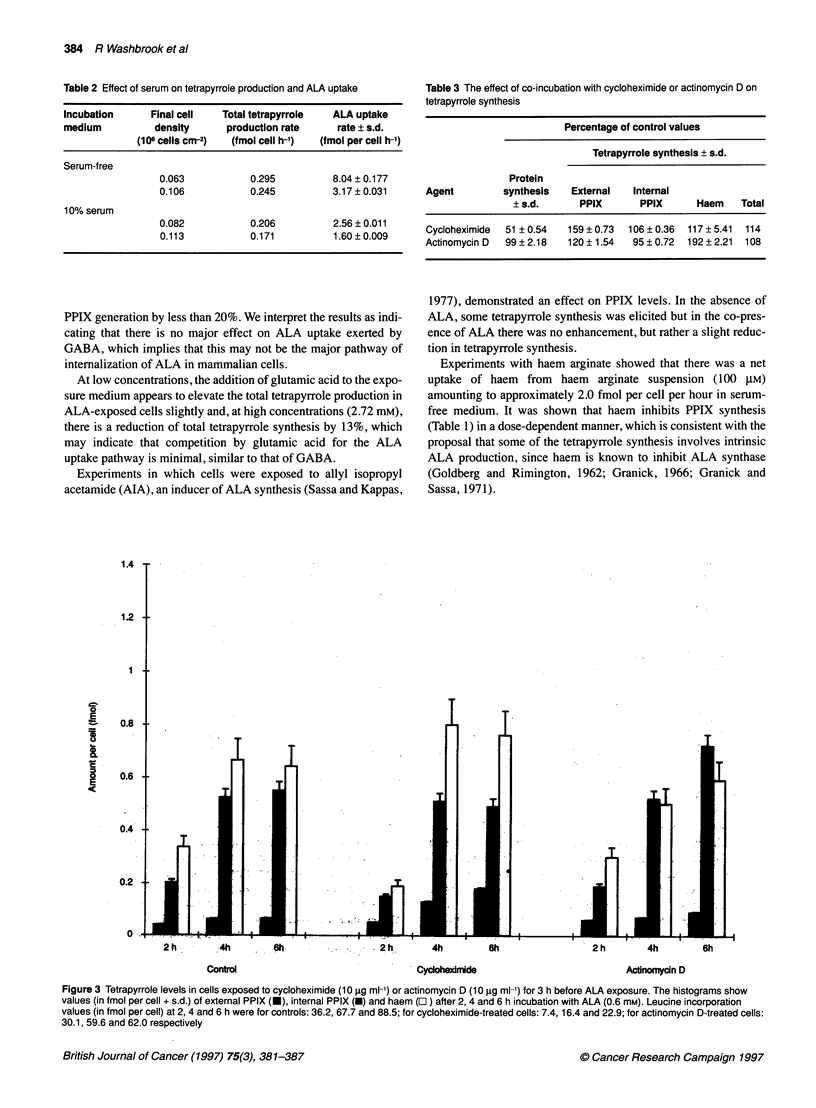
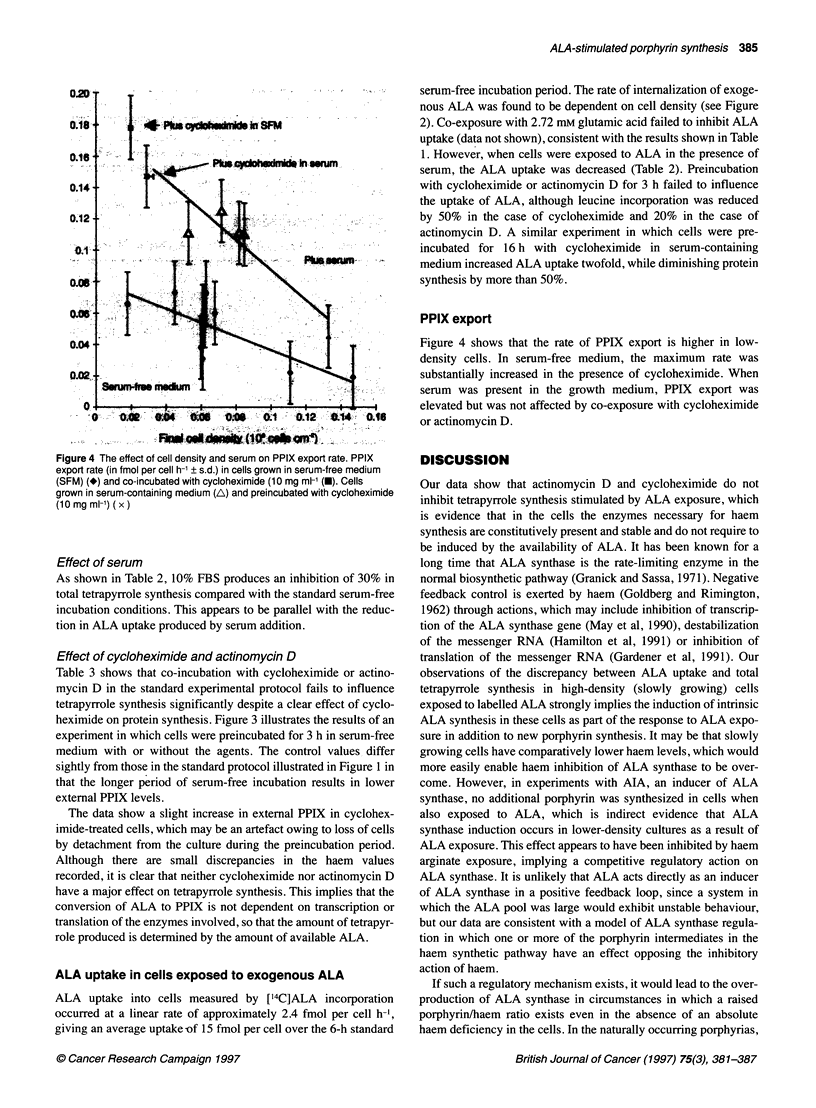
Tetrapyrrole synthesis in CNCM-1221 cells exposed to 0.6 mM aminolaevulinic acid (ALA) was found to be approximately linear over a 6-h period of incubation. The rate was not significantly affected by cell density over a range of 0.015 to 0.15 x 10(6) cells cm(-2) (final cell density). Tetrapyrrole synthesis was not affected by GABA or glutamic acid in concentrations up to 6 mM and 2.72 mM respectively, suggesting that these amino acids, which are similar in structure to ALA, do not competitively inhibit the ALA uptake pathway in these cells. Pre-exposure to haem arginate (up to 100 microM) was inhibitory, presumably by suppression (through the inhibition of ALA synthase) of an endogenous component of the response. The ALA-stimulated response was not modified by co-exposure to AIA (up to 100 mg ml(-1)). Despite significant reduction of protein synthesis, the porphyrinogenic response of cells exposed to ALA was unaffected by cycloheximide (10 microg ml(-1)) or actinomycin D (10 microg ml(-1)) even when cells were preincubated with these agents for 3 h before ALA exposure. Fetal bovine serum (10%) inhibited tetrapyrrole synthesis by 30% but increased the rate of porphyrin export by cells by a factor of 1.5. The uptake of [14C]ALA was shown to be strongly influenced by the density of the cultures. In dense cultures (final cell density of approximately 0.15 x 10(6) cells cm(-2)), the ALA uptake rate was less than 0.8 compared with a maximum rate of 4.2 fmol per cell h(-1) at a cell density of 0.02 x 10(6) cells cm(-2). Since tetrapyrrole synthesis is less affected than ALA uptake by cell density, the resultant discrepancy in ALA incorporation occurring in dense cultures implies that endogenous ALA synthesis is induced in these cells. ALA uptake was not affected by cycloheximide or actinomycin D in serum-free conditions. However, fetal bovine serum decreased external ALA uptake by about 50%. This effect was abrogated by preincubation with cycloheximide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedwell J., MacRobert A. J., Phillips D., Bown S. G. Fluorescence distribution and photodynamic effect of ALA-induced PP IX in the DMH rat colonic tumour model. Br J Cancer. 1992 Jun;65(6):818–824. doi: 10.1038/bjc.1992.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermúdez Moretti M., Correa García S. R., Chianelli M. S., Ramos E. H., Mattoon J. R., Batlle A. Evidence that 4-aminobutyric acid and 5-aminolevulinic acid share a common transport system into Saccharomyces cerevisiae. Int J Biochem Cell Biol. 1995 Feb;27(2):169–173. doi: 10.1016/1357-2725(95)00002-7. [DOI] [PubMed] [Google Scholar]
- Bermúdez Moretti M., Correa García S., Stella C., Ramos E., Batlle A. M. Delta-aminolevulinic acid transport in Saccharomyces cerevisiae. Int J Biochem. 1993 Dec;25(12):1917–1924. doi: 10.1016/0020-711x(88)90325-4. [DOI] [PubMed] [Google Scholar]
- Divaris D. X., Kennedy J. C., Pottier R. H. Phototoxic damage to sebaceous glands and hair follicles of mice after systemic administration of 5-aminolevulinic acid correlates with localized protoporphyrin IX fluorescence. Am J Pathol. 1990 Apr;136(4):891–897. [PMC free article] [PubMed] [Google Scholar]
- Fijan S., Hönigsmann H., Ortel B. Photodynamic therapy of epithelial skin tumours using delta-aminolaevulinic acid and desferrioxamine. Br J Dermatol. 1995 Aug;133(2):282–288. doi: 10.1111/j.1365-2133.1995.tb02630.x. [DOI] [PubMed] [Google Scholar]
- Fukuda H., Batlle A. M., Riley P. A. Kinetics of porphyrin accumulation in cultured epithelial cells exposed to ALA. Int J Biochem. 1993 Oct;25(10):1407–1410. doi: 10.1016/0020-711x(93)90689-c. [DOI] [PubMed] [Google Scholar]
- Fukuda H., Paredes S., Batlle A. M. Tumor-localizing properties of porphyrins. In vitro studies using the porphyrin precursor, aminolevulinic acid, in free and liposome encapsulated forms. Drug Des Deliv. 1989 Dec;5(2):133–139. [PubMed] [Google Scholar]
- Gardner L. C., Smith S. J., Cox T. M. Biosynthesis of delta-aminolevulinic acid and the regulation of heme formation by immature erythroid cells in man. J Biol Chem. 1991 Nov 15;266(32):22010–22018. [PubMed] [Google Scholar]
- Granick S., Sinclair P., Sassa S., Grieninger G. Effects by heme, insulin, and serum albumin on heme and protein synthesis in chick embryo liver cells cultured in a chemically defined medium, and a spectrofluorometric assay for porphyrin composition. J Biol Chem. 1975 Dec 25;250(24):9215–9225. [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Hamilton J. W., Bement W. J., Sinclair P. R., Sinclair J. F., Alcedo J. A., Wetterhahn K. E. Heme regulates hepatic 5-aminolevulinate synthase mRNA expression by decreasing mRNA half-life and not by altering its rate of transcription. Arch Biochem Biophys. 1991 Sep;289(2):387–392. doi: 10.1016/0003-9861(91)90428-l. [DOI] [PubMed] [Google Scholar]
- Iinuma S., Farshi S. S., Ortel B., Hasan T. A mechanistic study of cellular photodestruction with 5-aminolaevulinic acid-induced porphyrin. Br J Cancer. 1994 Jul;70(1):21–28. doi: 10.1038/bjc.1994.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegmair M., Baumgartner R., Knüchel R., Stepp H., Hofstädter F., Hofstetter A. Detection of early bladder cancer by 5-aminolevulinic acid induced porphyrin fluorescence. J Urol. 1996 Jan;155(1):105–110. [PubMed] [Google Scholar]
- Loh C. S., Vernon D., MacRobert A. J., Bedwell J., Bown S. G., Brown S. B. Endogenous porphyrin distribution induced by 5-aminolaevulinic acid in the tissue layers of the gastrointestinal tract. J Photochem Photobiol B. 1993 Sep;20(1):47–54. doi: 10.1016/1011-1344(93)80130-2. [DOI] [PubMed] [Google Scholar]
- Malik Z., Lugaci H. Destruction of erythroleukaemic cells by photoactivation of endogenous porphyrins. Br J Cancer. 1987 Nov;56(5):589–595. doi: 10.1038/bjc.1987.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May B. K., Bhasker C. R., Bawden M. J., Cox T. C. Molecular regulation of 5-aminolevulinate synthase. Diseases related to heme biosynthesis. Mol Biol Med. 1990 Oct;7(5):405–421. [PubMed] [Google Scholar]
- Muller-Eberhard U., Nikkilä H. Transport of tetrapyrroles by proteins. Semin Hematol. 1989 Apr;26(2):86–104. [PubMed] [Google Scholar]
- Rimington C. Haem biosynthesis and porphyrias: 50 years in retrospect. J Clin Chem Clin Biochem. 1989 Aug;27(8):473–486. [PubMed] [Google Scholar]
- Roberts D. J., Cairnduff F. Photodynamic therapy of primary skin cancer: a review. Br J Plast Surg. 1995 Sep;48(6):360–370. doi: 10.1016/s0007-1226(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Sassa S., Kappas A. Induction of aminolevulinate synthase and porphyrins in cultured liver cells maintained in chemically defined medium. Permissive effects of hormones on induction process. J Biol Chem. 1977 Apr 10;252(7):2428–2436. [PubMed] [Google Scholar]
- Schwartz S., Dahl J., Ellefson M., Ahlquist D. The "HemoQuant" test: a specific and quantitative determination of heme (hemoglobin) in feces and other materials. Clin Chem. 1983 Dec;29(12):2061–2067. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]


