Abstract
Interleukin 1alpha (IL-1alpha), Interleukin 6 (IL-6) and epidermal growth factor (EGF) were tested for their ability to regulate epithelial cervical cell cytokine production and secretion and to induce proliferation of human normal and neoplastic epithelial cervical cells. IL-1alpha, and IL-6 enhanced tumour and normal cell growth by 20-120%. The interleukins efficacy was similar to that of EGF for some cell lines but not for normal esocervical cells. The stimulatory effects of the interleukins were observed in both human papilloma virus (HPV)-infected and HPV-non-infected cervical cells. Normal cells constitutively expressed IL-1alpha, IL-6 and EGF mRNA. All cell lines except C33A expressed IL-1alpha mRNA. CaSki, C-4II and HT-3 expressed mRNA for IL-6. IL-1alpha induced or increased IL-6 mRNA levels in the Me-180 and HT-3 lines and in normal cervical cells. IL-6 induced: (1) the expression of its own mRNA only in Me-180 cells that constitutively lacked IL-6 mRNA; (2) the expression of IL-1alpha mRNA in C-33A and increased IL-1alpha mRNA level in the case of Me180 cells. Increased amounts of IL-6 mRNA were found in normal cells when treated with IL-1alpha. In spite of the pattern of mRNA expression, only HT-3 and normal cervical cells constitutively secreted IL-6, and only normal cells were able to produce IL-1alpha protein. A significant IL-1alpha-dependent increase of IL-6 secretion was found in Me-1 80, HT-3 and normal cells. IL-1alpha- and IL-6-driven cell proliferations were almost completely inhibited by the addition of neutralizing anti-IL-6 antibodies. Taken together, these data suggest that interleukins play a role in cervical carcinogenesis as autocrine and/or paracrine stimuli.
Full text
PDF
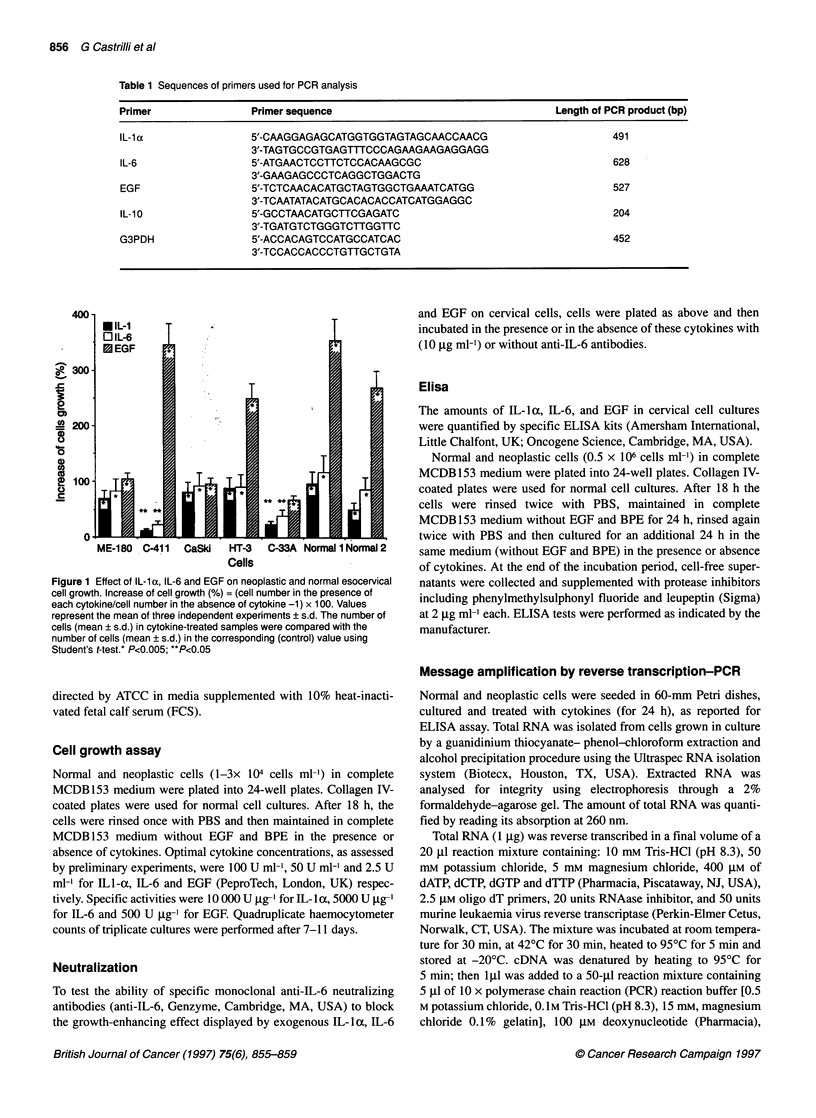
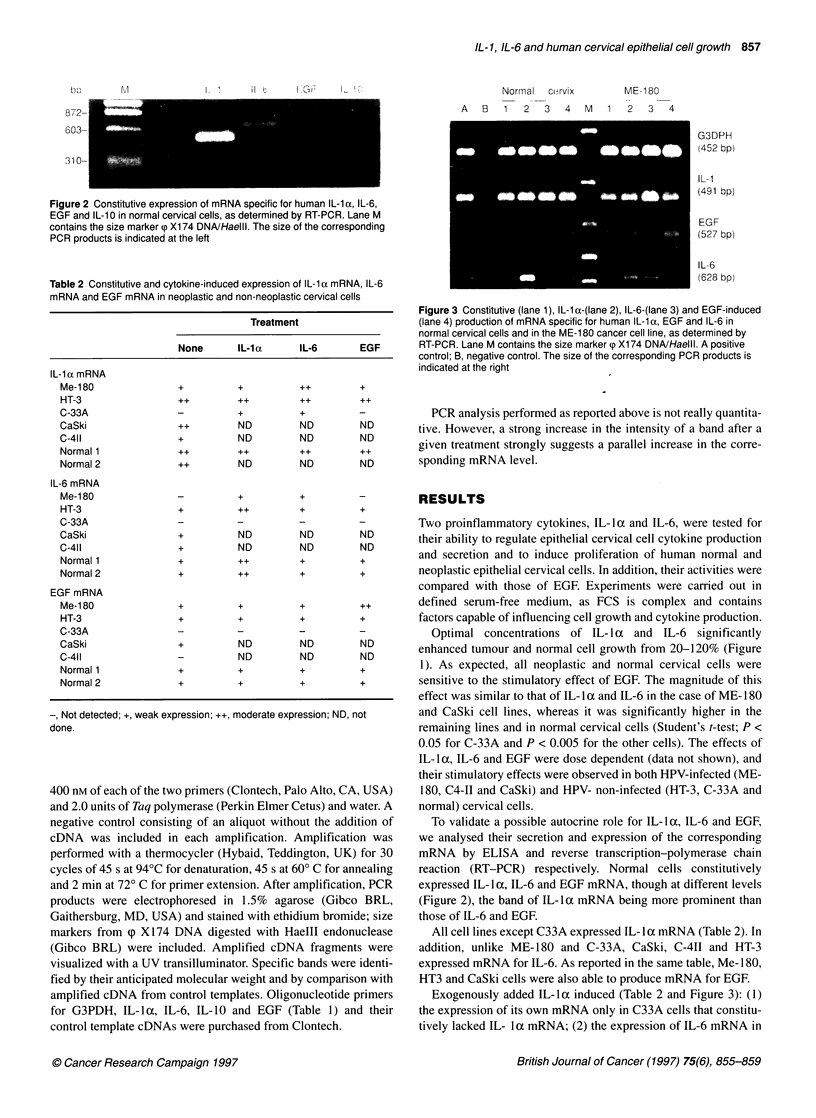

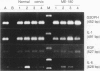
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breen E. C., Rezai A. R., Nakajima K., Beall G. N., Mitsuyasu R. T., Hirano T., Kishimoto T., Martinez-Maza O. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990 Jan 15;144(2):480–484. [PubMed] [Google Scholar]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992 Dec 15;52(24):6735–6740. [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M. The role of interleukin-1 in disease. N Engl J Med. 1993 Jan 14;328(2):106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- Elder J. T., Sartor C. I., Boman D. K., Benrazavi S., Fisher G. J., Pittelkow M. R. Interleukin-6 in psoriasis: expression and mitogenicity studies. Arch Dermatol Res. 1992;284(6):324–332. doi: 10.1007/BF00372034. [DOI] [PubMed] [Google Scholar]
- Eustace D., Han X., Gooding R., Rowbottom A., Riches P., Heyderman E. Interleukin-6 (IL-6) functions as an autocrine growth factor in cervical carcinomas in vitro. Gynecol Oncol. 1993 Jul;50(1):15–19. doi: 10.1006/gyno.1993.1156. [DOI] [PubMed] [Google Scholar]
- Gallo P., Frei K., Rordorf C., Lazdins J., Tavolato B., Fontana A. Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system: an evaluation of cytokines in cerebrospinal fluid. J Neuroimmunol. 1989 Jul;23(2):109–116. doi: 10.1016/0165-5728(89)90029-5. [DOI] [PubMed] [Google Scholar]
- Iglesias M., Plowman G. D., Woodworth C. D. Interleukin-6 and interleukin-6 soluble receptor regulate proliferation of normal, human papillomavirus-immortalized, and carcinoma-derived cervical cells in vitro. Am J Pathol. 1995 Apr;146(4):944–952. [PMC free article] [PubMed] [Google Scholar]
- Kawai K., Yamamoto M., Kameyama S., Kawamata H., Rademaker A., Oyasu R. Enhancement of rat urinary bladder tumorigenesis by lipopolysaccharide-induced inflammation. Cancer Res. 1993 Nov 1;53(21):5172–5175. [PubMed] [Google Scholar]
- Koutsky L. A., Holmes K. K., Critchlow C. W., Stevens C. E., Paavonen J., Beckmann A. M., DeRouen T. A., Galloway D. A., Vernon D., Kiviat N. B. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med. 1992 Oct 29;327(18):1272–1278. doi: 10.1056/NEJM199210293271804. [DOI] [PubMed] [Google Scholar]
- Maiman M., Fruchter R. G., Guy L., Cuthill S., Levine P., Serur E. Human immunodeficiency virus infection and invasive cervical carcinoma. Cancer. 1993 Jan 15;71(2):402–406. doi: 10.1002/1097-0142(19930115)71:2<402::aid-cncr2820710222>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Maiman M., Fruchter R. G., Serur E., Remy J. C., Feuer G., Boyce J. Human immunodeficiency virus infection and cervical neoplasia. Gynecol Oncol. 1990 Sep;38(3):377–382. doi: 10.1016/0090-8258(90)90077-x. [DOI] [PubMed] [Google Scholar]
- Miki S., Iwano M., Miki Y., Yamamoto M., Tang B., Yokokawa K., Sonoda T., Hirano T., Kishimoto T. Interleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett. 1989 Jul 3;250(2):607–610. doi: 10.1016/0014-5793(89)80805-1. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Martínez-Maza O., Hirano T., Breen E. C., Nishanian P. G., Salazar-Gonzalez J. F., Fahey J. L., Kishimoto T. Induction of IL-6 (B cell stimulatory factor-2/IFN-beta 2) production by HIV. J Immunol. 1989 Jan 15;142(2):531–536. [PubMed] [Google Scholar]
- O'Sullivan C., Lewis C. E., Harris A. L., McGee J. O. Secretion of epidermal growth factor by macrophages associated with breast carcinoma. Lancet. 1993 Jul 17;342(8864):148–149. doi: 10.1016/0140-6736(93)91348-p. [DOI] [PubMed] [Google Scholar]
- Pirisi L., Creek K. E., Doniger J., DiPaolo J. A. Continuous cell lines with altered growth and differentiation properties originate after transfection of human keratinocytes with human papillomavirus type 16 DNA. Carcinogenesis. 1988 Sep;9(9):1573–1579. doi: 10.1093/carcin/9.9.1573. [DOI] [PubMed] [Google Scholar]
- Schmauz R., Okong P., de Villiers E. M., Dennin R., Brade L., Lwanga S. K., Owor R. Multiple infections in cases of cervical cancer from a high-incidence area in tropical Africa. Int J Cancer. 1989 May 15;43(5):805–809. doi: 10.1002/ijc.2910430511. [DOI] [PubMed] [Google Scholar]
- Tartour E., Dorval T., Mosseri V., Deneux L., Mathiot C., Brailly H., Montero F., Joyeux I., Pouillart P., Fridman W. H. Serum interleukin 6 and C-reactive protein levels correlate with resistance to IL-2 therapy and poor survival in melanoma patients. Br J Cancer. 1994 May;69(5):911–913. doi: 10.1038/bjc.1994.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Cerami A. Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol. 1993;9:317–343. doi: 10.1146/annurev.cb.09.110193.001533. [DOI] [PubMed] [Google Scholar]
- Woodworth C. D., McMullin E., Iglesias M., Plowman G. D. Interleukin 1 alpha and tumor necrosis factor alpha stimulate autocrine amphiregulin expression and proliferation of human papillomavirus-immortalized and carcinoma-derived cervical epithelial cells. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2840–2844. doi: 10.1073/pnas.92.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth C. D., Simpson S. Comparative lymphokine secretion by cultured normal human cervical keratinocytes, papillomavirus-immortalized, and carcinoma cell lines. Am J Pathol. 1993 May;142(5):1544–1555. [PMC free article] [PubMed] [Google Scholar]
- Wu S., Rodabaugh K., Martinez-Maza O., Watson J. M., Silberstein D. S., Boyer C. M., Peters W. P., Weinberg J. B., Berek J. S., Bast R. C., Jr Stimulation of ovarian tumor cell proliferation with monocyte products including interleukin-1, interleukin-6, and tumor necrosis factor-alpha. Am J Obstet Gynecol. 1992 Mar;166(3):997–1007. doi: 10.1016/0002-9378(92)91379-o. [DOI] [PubMed] [Google Scholar]