Abstract
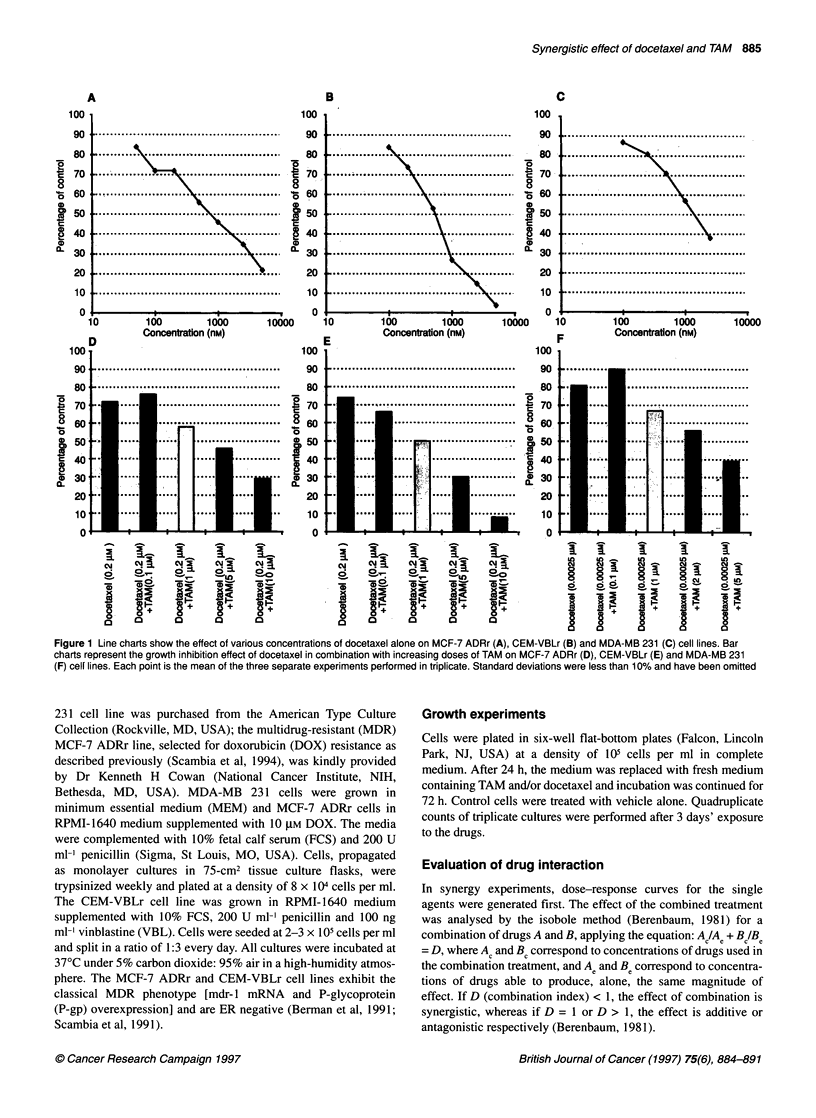
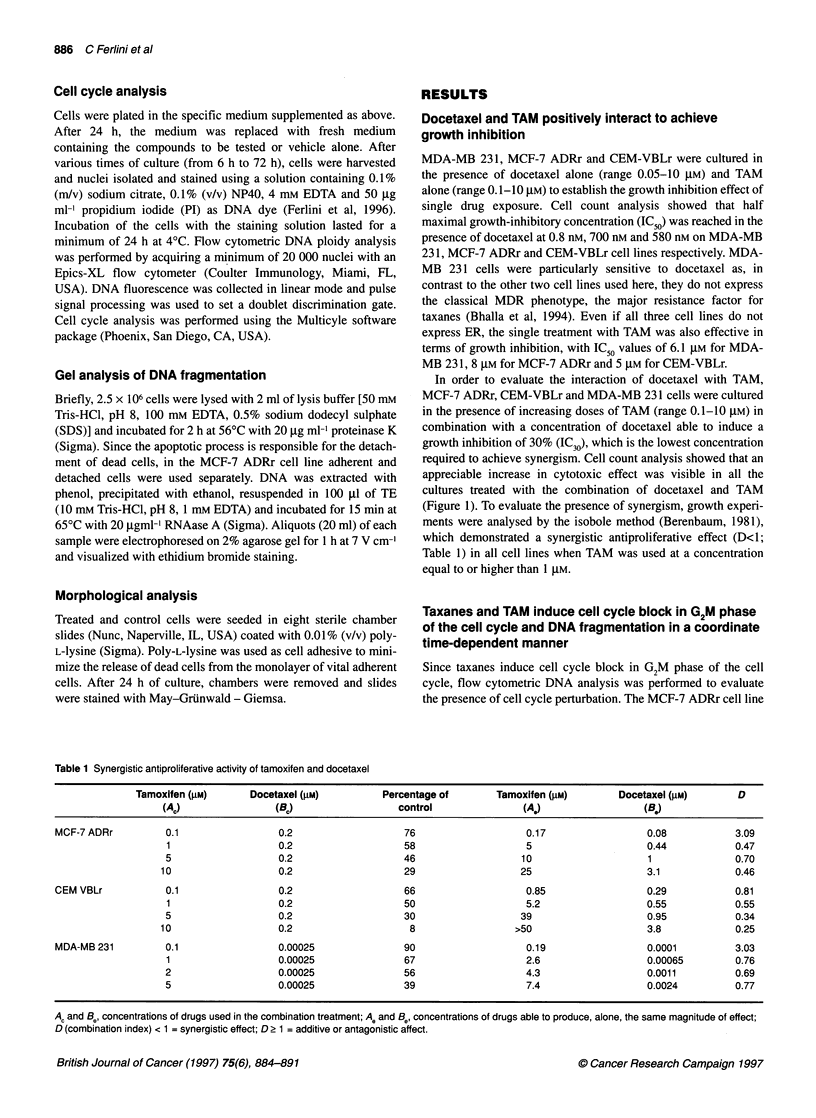
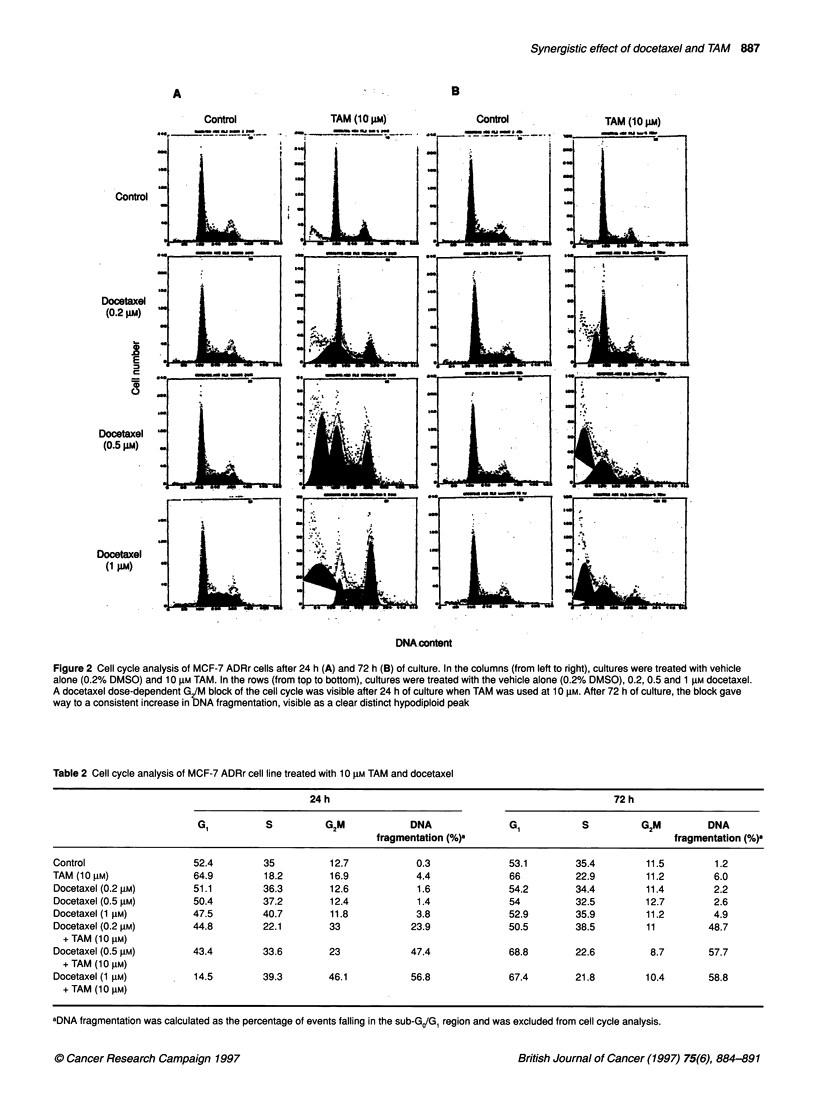
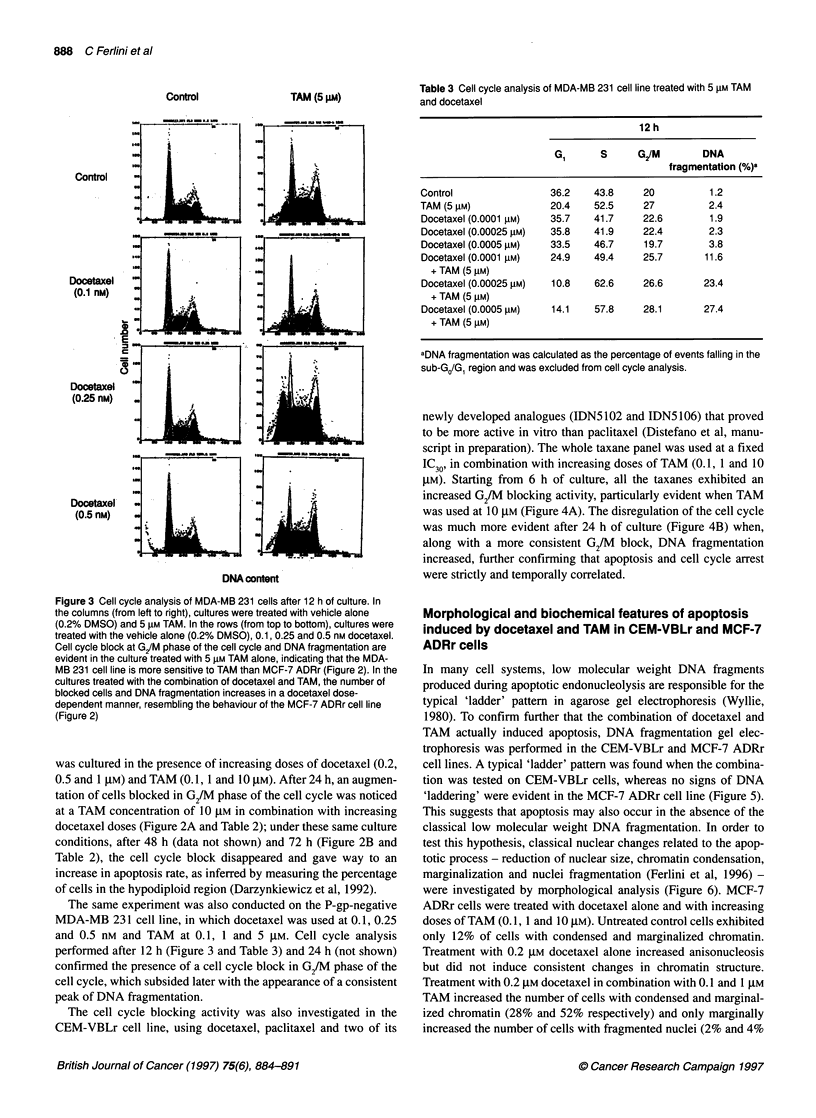
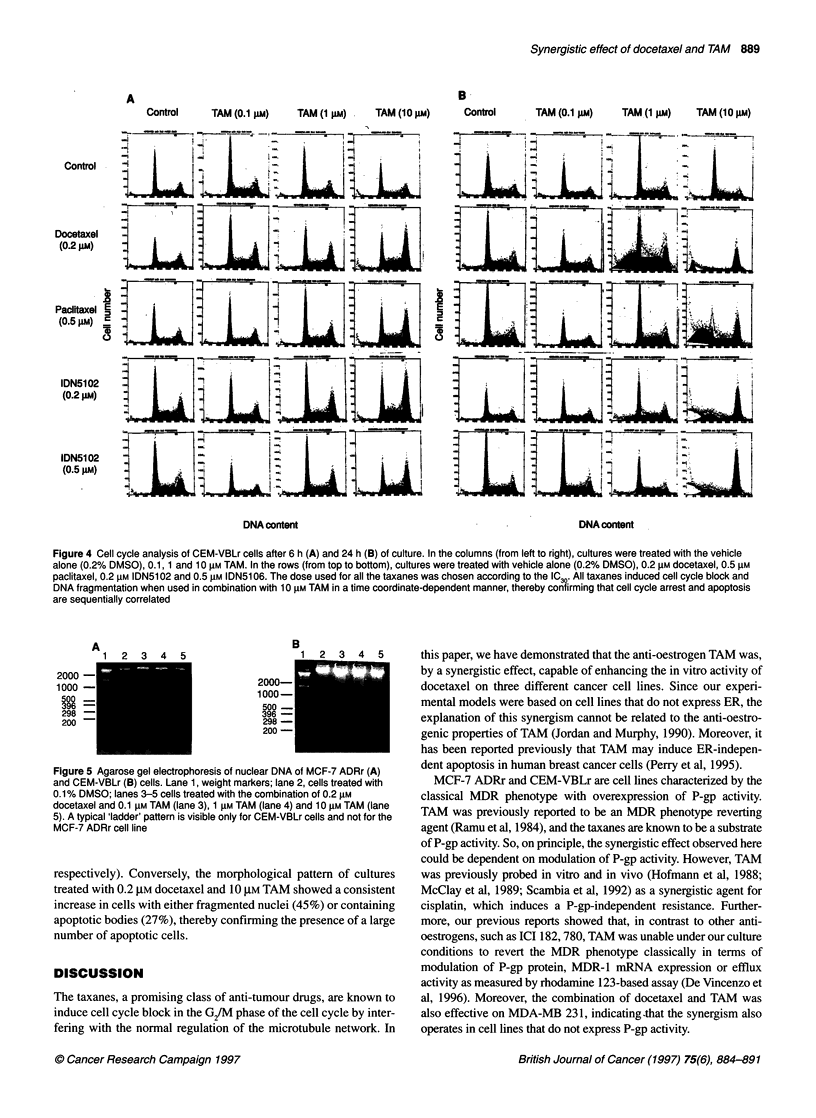
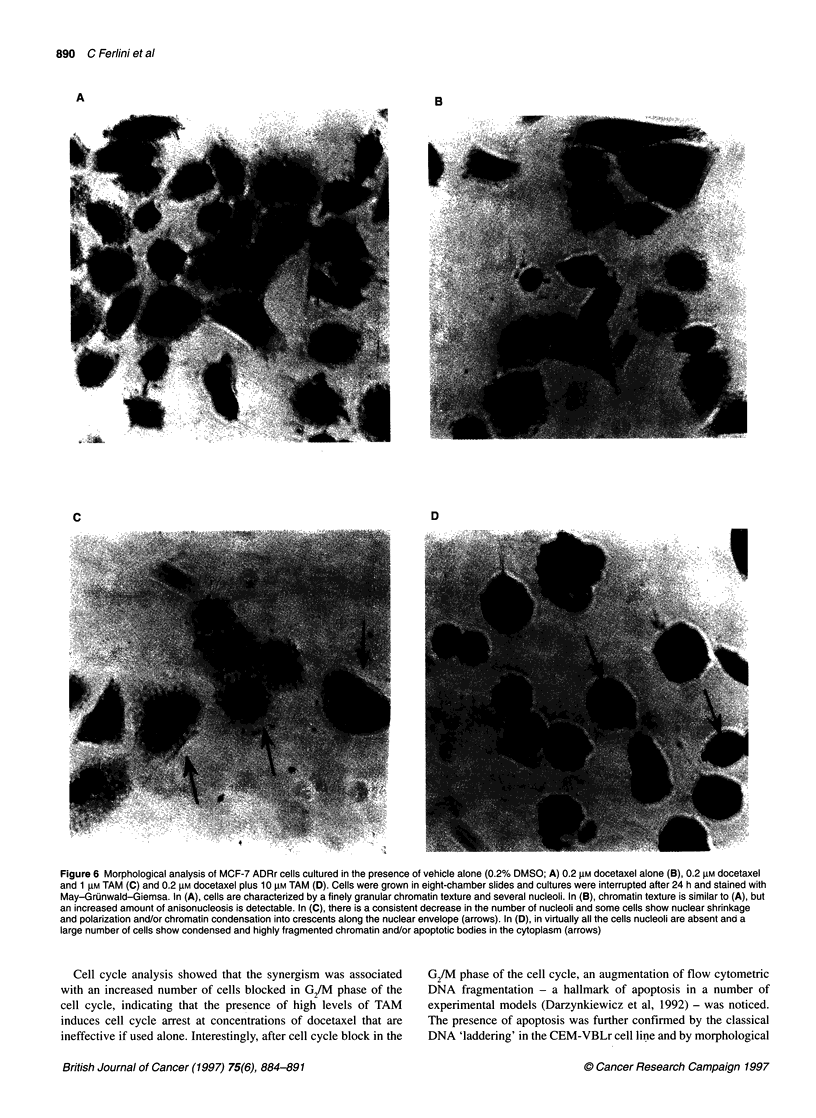
The taxanes are a promising family of anti-tumour drugs that block cell cycle replication by interfering with the microtubule network. The clinical use of these drugs involves some problems related to their low solubility and occurrence of resistance, which is mainly dependent on the multidrug-resistant (MDR) phenotype. To investigate the possible interaction between docetaxel and tamoxifen (TAM), three oestrogen receptor-negative cancer cell lines, MDR- MDA-MB 231, MDR + CEM-VBLr and MCF-7 ADRr, were used. In all three cell lines, the combination of docetaxel and TAM was more effective in terms of growth inhibition than single drug exposure. Isobolic analysis confirmed the presence of synergism in all cell lines when docetaxel was used at 0.2 microM and TAM at a dose equal to or higher than 1 microM. Flow cytometric DNA analysis performed on the three cell lines showed that TAM was able to increase the G2/M blocking activity of docetaxel. This blocking activity was followed by an increased flow cytometric DNA fragmentation suggestive of the presence of apoptosis, which was confirmed by DNA gel fragmentation and morphological analysis. While an antagonistic effect on P-glycoprotein (P-gp) activity may contribute to the synergistic effect of tamoxifen and docetaxel on CEM-VBLr and MCF-7 ADRr, other mechanisms must be involved, as the synergistic effect is also apparent with a P-gp-negative cell line.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardon S., Vignon F., Montcourrier P., Rochefort H. Steroid receptor-mediated cytotoxicity of an antiestrogen and an antiprogestin in breast cancer cells. Cancer Res. 1987 Mar 1;47(5):1441–1448. [PubMed] [Google Scholar]
- Berenbaum M. C. Criteria for analyzing interactions between biologically active agents. Adv Cancer Res. 1981;35:269–335. doi: 10.1016/s0065-230x(08)60912-4. [DOI] [PubMed] [Google Scholar]
- Berman E., Adams M., Duigou-Osterndorf R., Godfrey L., Clarkson B., Andreeff M. Effect of tamoxifen on cell lines displaying the multidrug-resistant phenotype. Blood. 1991 Feb 15;77(4):818–825. [PubMed] [Google Scholar]
- Bhalla K., Huang Y., Tang C., Self S., Ray S., Mahoney M. E., Ponnathpur V., Tourkina E., Ibrado A. M., Bullock G. Characterization of a human myeloid leukemia cell line highly resistant to taxol. Leukemia. 1994 Mar;8(3):465–475. [PubMed] [Google Scholar]
- Darzynkiewicz Z., Bruno S., Del Bino G., Gorczyca W., Hotz M. A., Lassota P., Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13(8):795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- Ferlini C., Di Cesare S., Rainaldi G., Malorni W., Samoggia P., Biselli R., Fattorossi A. Flow cytometric analysis of the early phases of apoptosis by cellular and nuclear techniques. Cytometry. 1996 Jun 1;24(2):106–115. doi: 10.1002/(SICI)1097-0320(19960601)24:2<106::AID-CYTO2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hofmann J., Doppler W., Jakob A., Maly K., Posch L., Uberall F., Grunicke H. H. Enhancement of the antiproliferative effect of cis-diamminedichloroplatinum(II) and nitrogen mustard by inhibitors of protein kinase C. Int J Cancer. 1988 Sep 15;42(3):382–388. doi: 10.1002/ijc.2910420313. [DOI] [PubMed] [Google Scholar]
- Issandou M., Faucher C., Bayard F., Darbon J. M. Opposite effects of tamoxifen on in vitro protein kinase C activity and endogenous protein phosphorylation in intact MCF-7 cells. Cancer Res. 1990 Sep 15;50(18):5845–5850. [PubMed] [Google Scholar]
- Leonessa F., Jacobson M., Boyle B., Lippman J., McGarvey M., Clarke R. Effect of tamoxifen on the multidrug-resistant phenotype in human breast cancer cells: isobologram, drug accumulation, and M(r) 170,000 glycoprotein (gp170) binding studies. Cancer Res. 1994 Jan 15;54(2):441–447. [PubMed] [Google Scholar]
- Lien E. A., Solheim E., Ueland P. M. Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res. 1991 Sep 15;51(18):4837–4844. [PubMed] [Google Scholar]
- Lopes M. C., Vale M. G., Carvalho A. P. Ca2(+)-dependent binding of tamoxifen to calmodulin isolated from bovine brain. Cancer Res. 1990 May 1;50(9):2753–2758. [PubMed] [Google Scholar]
- Mastropaolo D., Camerman A., Luo Y., Brayer G. D., Camerman N. Crystal and molecular structure of paclitaxel (taxol). Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6920–6924. doi: 10.1073/pnas.92.15.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClay E. F., Mastrangelo M. J., Sprandio J. D., Bellet R. E., Berd D. The importance of tamoxifen to a cisplatin-containing regimen in the treatment of metastatic melanoma. Cancer. 1989 Apr 1;63(7):1292–1295. doi: 10.1002/1097-0142(19890401)63:7<1292::aid-cncr2820630711>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Perry R. R., Kang Y., Greaves B. R. Relationship between tamoxifen-induced transforming growth factor beta 1 expression, cytostasis and apoptosis in human breast cancer cells. Br J Cancer. 1995 Dec;72(6):1441–1446. doi: 10.1038/bjc.1995.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramu A., Glaubiger D., Fuks Z. Reversal of acquired resistance to doxorubicin in P388 murine leukemia cells by tamoxifen and other triparanol analogues. Cancer Res. 1984 Oct;44(10):4392–4395. [PubMed] [Google Scholar]
- Scambia G., Ranelletti F. O., Benedetti Panici P., Piantelli M., Bonanno G., De Vincenzo R., Ferrandina G., Pierelli L., Capelli A., Mancuso S. Quercetin inhibits the growth of a multidrug-resistant estrogen-receptor-negative MCF-7 human breast-cancer cell line expressing type II estrogen-binding sites. Cancer Chemother Pharmacol. 1991;28(4):255–258. doi: 10.1007/BF00685531. [DOI] [PubMed] [Google Scholar]
- Scambia G., Ranelletti F. O., Benedetti Panici P., Piantelli M., De Vincenzo R., Bonanno G., Ferrandina G., Isola G., Mancuso S. Synergistic antiproliferative activity of tamoxifen and cisplatin on primary ovarian tumours. Eur J Cancer. 1992;28A(11):1885–1889. doi: 10.1016/0959-8049(92)90029-2. [DOI] [PubMed] [Google Scholar]
- Scambia G., Ranelletti F. O., Panici P. B., De Vincenzo R., Bonanno G., Ferrandina G., Piantelli M., Bussa S., Rumi C., Cianfriglia M. Quercetin potentiates the effect of adriamycin in a multidrug-resistant MCF-7 human breast-cancer cell line: P-glycoprotein as a possible target. Cancer Chemother Pharmacol. 1994;34(6):459–464. doi: 10.1007/BF00685655. [DOI] [PubMed] [Google Scholar]
- Schiff P. B., Fant J., Horwitz S. B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979 Feb 22;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Slichenmyer W. J., Von Hoff D. D. Taxol: a new and effective anti-cancer drug. Anticancer Drugs. 1991 Dec;2(6):519–530. [PubMed] [Google Scholar]
- Trump D. L., Smith D. C., Ellis P. G., Rogers M. P., Schold S. C., Winer E. P., Panella T. J., Jordan V. C., Fine R. L. High-dose oral tamoxifen, a potential multidrug-resistance-reversal agent: phase I trial in combination with vinblastine. J Natl Cancer Inst. 1992 Dec 2;84(23):1811–1816. doi: 10.1093/jnci/84.23.1811. [DOI] [PubMed] [Google Scholar]
- Wärri A. M., Huovinen R. L., Laine A. M., Martikainen P. M., Härkönen P. L. Apoptosis in toremifene-induced growth inhibition of human breast cancer cells in vivo and in vitro. J Natl Cancer Inst. 1993 Sep 1;85(17):1412–1418. doi: 10.1093/jnci/85.17.1412. [DOI] [PubMed] [Google Scholar]