Abstract
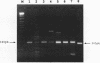
An internal fragment of the recA gene from eight gram-positive organisms has been amplified by using degenerate primers in a polymerase chain reaction. The internal 348- or 360-bp recA DNA segments from Bacillus subtilis, Clostridium acetobutylicum, Lactobacillus bulgaricus, Lactobacillus helveticus, Leuconostoc mesanteroides, Listeria monocytogenes, Staphylococcus aureus, and Streptococcus salivarus subsp. thermophilus were amplified, cloned, and sequenced. The G + C contents of the DNA from these species range from 28 to 52%. The sequences of the bacterial recA genes show strong relatedness. This method is particularly useful for the recovery of the recA genes of gram-positive bacteria and avoids the difficulties of using a genetic complementation test for cloning.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988 Nov 25;16(22):10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. O., Sedgwick S. G., Colston M. J. Novel structure of the recA locus of Mycobacterium tuberculosis implies processing of the gene product. J Bacteriol. 1991 Sep;173(18):5653–5662. doi: 10.1128/jb.173.18.5653-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybvig K., Hollingshead S. K., Heath D. G., Clewell D. B., Sun F., Woodard A. Degenerate oligonucleotide primers for enzymatic amplification of recA sequences from gram-positive bacteria and mycoplasmas. J Bacteriol. 1992 Apr;174(8):2729–2732. doi: 10.1128/jb.174.8.2729-2732.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybvig K., Woodard A. Cloning and DNA sequence of a mycoplasmal recA gene. J Bacteriol. 1992 Feb;174(3):778–784. doi: 10.1128/jb.174.3.778-784.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohavong P., Thilly W. G. Fidelity of DNA polymerases in DNA amplification. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9253–9257. doi: 10.1073/pnas.86.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero R., Yasbin R. E. Cloning of the Bacillus subtilis recE+ gene and functional expression of recE+ in B. subtilis. J Bacteriol. 1988 Jan;170(1):335–344. doi: 10.1128/jb.170.1.335-344.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. V., Kokjohn T. A. General microbiology of recA: environmental and evolutionary significance. Annu Rev Microbiol. 1990;44:365–394. doi: 10.1146/annurev.mi.44.100190.002053. [DOI] [PubMed] [Google Scholar]
- Roca A. I., Cox M. M. The RecA protein: structure and function. Crit Rev Biochem Mol Biol. 1990;25(6):415–456. doi: 10.3109/10409239009090617. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Kapelner S., Sommer S. S. Formamide can dramatically improve the specificity of PCR. Nucleic Acids Res. 1990 Dec 25;18(24):7465–7465. doi: 10.1093/nar/18.24.7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story R. M., Steitz T. A. Structure of the recA protein-ADP complex. Nature. 1992 Jan 23;355(6358):374–376. doi: 10.1038/355374a0. [DOI] [PubMed] [Google Scholar]
- Yasbin R. E., Stranathan M., Bayles K. W. The recE(A)+ gene of B subtilis and its gene product: further characterization of this universal protein. Biochimie. 1991 Feb-Mar;73(2-3):245–250. doi: 10.1016/0300-9084(91)90209-j. [DOI] [PubMed] [Google Scholar]