Abstract
Purpose
The Children's Visual Function Questionnaire (CVFQ) is a vision-specific quality-of-life instrument designed for use with children up to 7 years of age. The goal of this study was to assess the construct validity of CVFQ subscales by examining their sensitivity to four research questions commonly posed in ophthalmic clical trials.
Methods
CVFQ Competence, Personality, Family Impact, and Treatment Difficulty subscale scores were compared for groups of pediatric patients with unilateral vs. bilateral disease (35 bilateral and 38 unilateral cataract), different severity of visual impairment (61 ROP grouped by acuity), difficulty of treatment regimen (22 optical, 44 surgery, 38 surgery+optical, 35 surgery+optical+occlusion), and alternative treatments for the same condition (24 IOLs, 37 aphakic contact lenses).
Results
Patients treated for bilateral cataracts had significantly worse Competence subscale scores than patients treated for unilateral cataract, and patients with bilateral severe ROP (visual acuity 20/200 or poorer in both eyes) had significantly worse Competence subscale scores than other patients with ROP. Treatment Difficulty Subscale scores were worse for patients with unilateral cataracts than for those with bilateral cataracts, for patients treated with occlusion therapy than for those treated with surgery or optical correction, and for those treated with an aphakic contact lens than for those treated with an IOL. Family Impact Subscale scores were worse for patients with unilateral cataracts than for those with bilateral cataracts, for patients treated with surgery than for those treated with optical correction, and for those with severe bilateral ROP than for any other patients with ROP.
Conclusion
The CVFQ subscales quantified meaningful differences among pediatric patient groups who were chosen to address key research questions commonly posed in ophthalmic clinical trials.
The Children's Visual Function Questionnaire (CVFQ) is a vision-specific quality-of-life instrument designed for use with parents of infants and young children up to 7 years of age. 1 Age-specific versions of the CVFQ are available for younger (<3 years of age; 34 items) and older children (3-7 years of age; 39 items). The CVFQ contains 4 quality-of-life-related subscales: Competence, Personality, Family Impact, and Treatment Difficulty. Felius et al. 1 assessed the relationship between subscale scores and clinical measures (visual acuity and diagnosis) and the internal consistency and reliability of the subscales for a cohort of 773 pediatric patients with a wide variety of ophthalmic disorders. Cronbach's α 2, a coefficient for internal consistency of the items in a subscale to assesses how well the set of questions reflect a single underlying construct , ranged from 0.60 to 0.81 in the younger age group and from 0.76 to 0.86 in the older age group.
In a follow-up study, item-response analysis (Rasch analysis) showed that the items are well distributed along the Rasch difficulty scale [Felius et al. (2004) Abstract, AAPOS annual meeting], suggesting little contamination by factors other than the effects of vision problems. Rasch scores correlated well with acuity deficits as well as with the total CVFQ score. Rasch analysis also revealed that the CVFQ is most sensitive to quality of life effects among patients with moderate to severe visual impairment (≥0.4 logMAR below normal).
The goal of this study was to further evaluate this instrument by assessing the construct validity of the CVFQ subscales. In general, construct validity of a quality-of-life instrument refers to its ability to measure what it is purported to measure, and can be assessed in numerous ways. Here, we chose to examine the sensitivity of the subscales to meaningful differences among pediatric patient groups. We evaluated four key research questions that are commonly posed in ophthalmic clinical trials. First, do CVFQ subscale scores differ for pediatric patients with unilateral vs. bilateral visual impairment? Second, do CVFQ subscale scores reflect differences in severity of visual impairment due to a single ophthalmic disorder? Third, do CVFQ subscale scores differ among patients participating in treatment regimens of varying degrees of difficulty? Fourth, do CVFQ subscale scores reflect differences between patients who had alternative treatments for the same ophthalmic disorder? Based on the same data used to address these 4 questions, we were also able to examine the test-retest reliability of subscale scores and the responsiveness of the subscale scores to changes in treatment regimens.
Subjects and Methods
Subjects
Families of infants and children with infantile esotropia (n=38), refractive error (n=22), a history of visually significant cataract(s) (n=73), or a history of retinopathy of prematurity (ROP; n=61), ≤ 7 years of age, who were referred to the Retina Foundation of the Southwest for evaluation of visual acuity and/or stereopsis were invited to participate in the study. Families of patients with multiple eye disorders, systemic disease, or neurological disorders were not invited to participate. Children who were not accompanied by an English–speaking parent involved in daily care of the child were not eligible. Of the eligible families, all but one agreed to participate. Written informed consent was obtained from one or both parents prior to the patient's participation. A medical record release form was signed by the parent to permit review of ophthalmic findings. This research protocol observed the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center.
CVFQ and Visual Acuity
For patients under age 3 years, the CVFQ version for younger children was used, and for patients age 3 years and older, the CVFQ version for older children was used. The written questionnaire was completed by a parent in an unsupervised waiting room. For each question, the parents chose one response option from a 5-point Likert-type scale or chose “nor applicable to my child”. Instructions were included on the front page of the questionnaire. Proxy assessments of children's vision-related quality of life by their parents has been validated [Odom et al. (2004) ARVO abstract].
Each response to an item in the questionnaire corresponded to a score between 1 (“best”) and 0 (“worst”). Competence, Personality, Family Impact, and Treatment Difficulty subscale scores were defined as the average of the scores of those items that belong to each subscale. As a result, all subscale scores also ranged from 1 (“best”) to 0 (“worst”); i.e., from most to least competent, from most pleasant and sociable to least pleasant and sociable personality, from least to most family impact, and from least to most treatment difficulty. A Total Score was computed by taking the average of all subscales. We demonstrated earlier that subscale scores (as well as Total Scores) from the younger and older age groups can be combined 1.
Visual acuity and the CVFQ were assessed during the same visit. Visual acuity was assessed using Teller Acuity Cards 3 for children <3 years of age or with crowded HOTV 4 optotypes for children ≥ 3 years of age.
Clinical Variables
Clinical variables gathered through patient interview and medical record review included diagnostic group (infantile esotropia, accommodative esotropia, anisometropia, cataract, or ROP) and current treatment regimen (glasses, occlusion, atropine, contact lenses, IOL, other).
Analyses
Patient groups compared in addressing each of the 4 questions are summarized in Table 1. Subscale scores from patients who had been treated for visually significant cataracts were utilized to examine the sensitivity of the CVFQ to unilateral versus bilateral visual impairment. Subscale scores from a heterogeneous cohort of patients with regressed or cicatricial ROP were utilized to examine the sensitivity of the CVFQ to severity of visual impairment; these patients were grouped into five visual acuity outcome subgroups: 20/200 or poorer in both eyes (severe bilateral visual impairment), 20/200 or worse in one eye with 20/50-20/125 in the fellow eye, 20/50-20/125 in both eyes, 20/50-20/125 in one eye and 20/40-20/20 in the fellow eye, or 20/40-20/20 in both eyes (normal visual acuity). Subscale scores from a heterogeneous cohort of patients treated for anisometropia, esotropia, or cataracts were utilized to examine the sensitivity of the CVFQ to differences in difficulty of treatment regimens. Subscale scores from patients who had been treated for visually significant unilateral cataracts with either aphakic contact lenses or IOLs were utilized to examine the sensitivity of the CVFQ to alternative treatments for a single ophthalmic disorder. Group results were analyzed with ANOVAs and post-hoc Scheffé tests.
Table 1.
Patient Groups for Each Comparison
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|
|
Unilateral vs. bilateral disease |
Visually significant unilateral cataract |
Visually significant bilateral cataract |
|||
|
Severity of visual impairment in a single ophthalmic disorder |
ROP 20/200 or poorer in both eyes |
ROP 20/200 or poorer in one eye, 20/50- 20/125 in fellow eye |
ROP 20/50- 20/125 in both eyes |
ROP 20/50-20/125 in one eye, 20/20-20/40 in fellow eye |
ROP 20/20-20/40 in both eyes |
|
Different treatment regimens |
Optical correction (accommodative ET, anisometropia) |
Surgery (ET) |
Optical correction and surgery (ET, cataracts) |
Optical correction, surgery, and occlusion therapy (ET, cataracts) |
|
|
Alternative treatments for a single ophthalmic disorder |
Unilateral cataracts optically corrected with IOLs |
Unilateral cataracts optically corrected with aphakic contact lenses |
Abbreviations: ROP=retinopathy of prematurity; ET=esotropia; IOL=intraocular lens.
Some patients had multiple visits, and we were able to assess CVFQ test-retest reliability. Visual acuity of each eye on each visit was coded as normal (within the normal tolerance limits for the age), borderline (within 0.1 logMAR of the normal tolerance limit), mildly impaired (0.2 to 0.3 logMAR below the normal tolerance limit), moderately impaired (0.4 to 0.9 logMAR below the normal tolerance limit) or severely impaired (≥1.0 logMAR below the normal tolerance limit). We identified 52 patients who had two questionnaires completed within 6 months (mean±SD = 4.4±1.7 months) with no change in visual acuity category for either eye between the two visits. This test-retest data set was analyzed using the method described by Bland and Altman 2.
Other patients (n=85) with multiple visits had a change in treatment regimen between visits. This change allowed us to assess whether changes in the Treatment Difficulty subscale scale scores occurred as a result of changes in treatment. Difference scores for the Treatment Difficulty subscale were computed for each patient and t-tests were used to determine whether the mean difference score differed significantly from zero.
Results
Unilateral versus Bilateral Visual Impairment
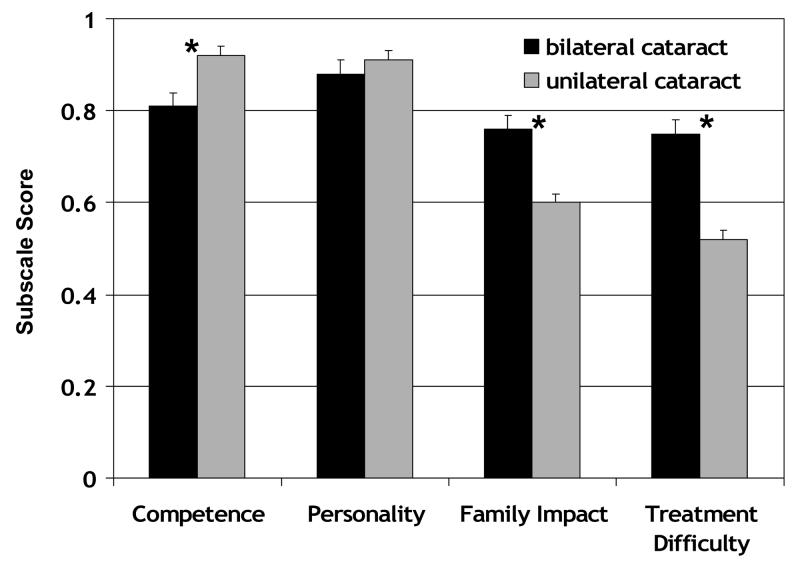
CVFQ subscale scores for 73 patients treated for visually significant cataracts are summarized in Figure 1. All patients had cataract(s) extracted and were wearing optical correction (glasses, contact lenses, or IOLs). There was no significant difference in the age distributions in the unilateral and bilateral groups (p=0.82). The mean (±SD) of the unilateral group was 2.2±1.8 years (range: 0.3 to 6.4 years) and of the bilateral group was 2.2±1.6 years (range: 0.3 to 5.8 years). There was no significant difference in the visual acuity of the affected eyes in the unilateral and bilateral groups (p=0.44).
Figure 1.
CVFQ subscale scores (mean ± standard error) for pediatric patients treated for visually significant bilateral (n=35) or unilateral cataracts (n=38). Asterisks indicate pairwise comparisons that were significant on post-hoc Scheffé tests.
Patients treated for bilateral cataracts had significantly poorer Competence subscale scores than patients treated for unilateral cataracts (p<0.001). The mean (±SD) of the unilateral group was 0.68±0.46 logMAR (range: 0.10 to 2.00 logMAR) and of the bilateral group was 0.60±0.40 logMAR (range: 0.10 to 1.83 logMAR). Patients treated for unilateral cataracts had significantly poorer Family Impact and Treatment Difficulty subscale scores than patients treated for bilateral cataracts (p<0.001 for both subscales). Personality subscale scores, which ranged from 0.40 to 1.0, did not differ significantly (p=0.34).
Severity of Visual Impairment
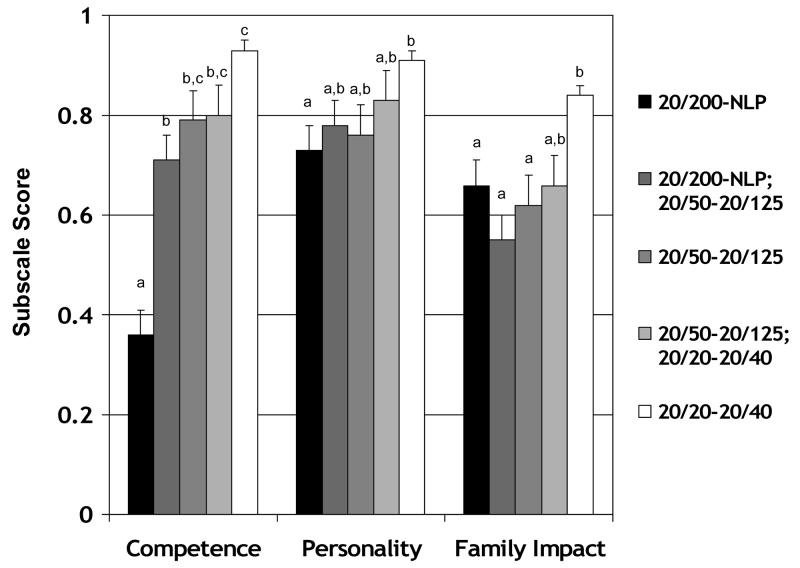
CVFQ subscale scores for 61 patients with retinopathy of prematurity (ROP) who were 3 to 7 years of age at the time of questionnaire administration are summarized in Figure 2. There were no significant differences among the age distributions for the five acuity groups (for all pairwise comparisons, t≤0.64, p≥0.53. The means (±SD) of the five groups, respectively, were 2.2±1.7 years, 2.5±1.8 years, 2.1±1.6 years, 2.7±1.8 years, and 2.5±1.9 years. One-way ANOVA identified a significant effect of visual outcome category on Competence, Personality, and Family Impact subscale scores. Post-hoc Scheffé tests were used to make pairwise comparisons among visual acuity outcome groups. Patients with ROP and severe bilateral visual impairment had significantly poorer Competence subscale scores than all other groups of patients with ROP (p<0.001 for all pairwise comparisons). Patients with ROP and normal visual acuity had significantly better Competence subscale scores than patients with ROP and visual acuity of 20/125 in one eye and 20/200 or poorer in the fellow eye ( p<0.01). Patients with ROP and normal visual acuity had significantly better Personality subscale scores than patients with severe bilateral visual impairment(p<0.01 for all pairwise comparisons). Patients with ROP and normal visual acuity had significantly better Family Impact subscale scores than patients with ROP and visual acuity of 20/125 or poorer ( p<0.04 for all pairwise comparisons). Treatment Difficulty subscale scores were not evaluated in this group of patients with ROP since only 56% were receiving treatment at the time of their visit.
Figure 2.
CVFQ subscale scores (mean ± standard error) for pediatric patients age 3 to 7 years with regressed or cicatricial retinopathy of prematurity and acuity of 20/200 or poorer in both eyes (n=11), 20/200 or poorer in one eye and 20/50-20/125 in the fellow eye (n=10), 20/50-20/125 in both eyes (n=8), 20/50-20/125 in one eye and 20/20-20/40 in the fellow eye (n=10), or 20/20-20/40 in both eyes (n=22). Bars with different superscripts are significantly different from each other.
Treatment Regimen
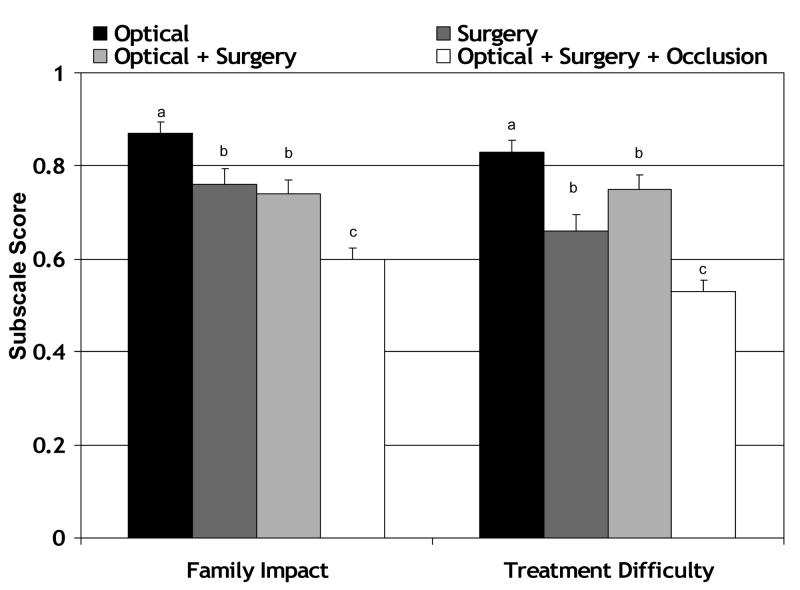
Whether subscale scores reflect the relative difficulty of various treatment regimens was examined in two ways. First, Family Impact and Treatment Difficulty subscale scores obtained from 133 patients with anisometropia, esotropia, or cataract experiencing different treatment regimens just prior to the visit at which the questionnaire was completed were evaluated (Figure 3). There were no significant differences among the age distributions for the four treatment groups (for all pairwise comparisons, p≥0.82. The means (±SD) of the four groups, respectively, were 2.2±1.1 years, 2.2±1.6 years, 2.2±1.8 years, and 2.2±1.6 years. One-way ANOVA identified a significant effect of treatment regimen on Family Impact and Treatment Difficulty subscale scores (p<0.001 for both subscales). Post-hoc Scheffé tests were used to make pairwise comparisons among treatment regimens. Patients treated with optical correction alone had significantly better Family Impact subscale scores than any of the other treatment groups (p<0.006 for all pairwise comparisons). Patients treated with optical correction alone had significantly better Treatment Difficulty subscale scores than patients treated with optical correction + surgery (p<0.001). Patients treated with surgery alone had significantly better Family Impact subscale scores than patients treated with optical correction + surgery (p<0.001). Patients treated with optical correction + surgery had significantly better Family Impact and Treatment Difficulty subscale scores than patients treated with optical correction + surgery + occlusion (p<0.001 for both pairwise comparisons).
Figure 3.
CVFQ subscale scores (mean ± standard error) as a function of difficulty of treatment regimen, including optical correction (n=22), surgery (n=44), surgery and optical correction (n=38), and surgery, optical correction, and occlusion therapy (n=35). Bars with different superscripts are significantly different from each other.
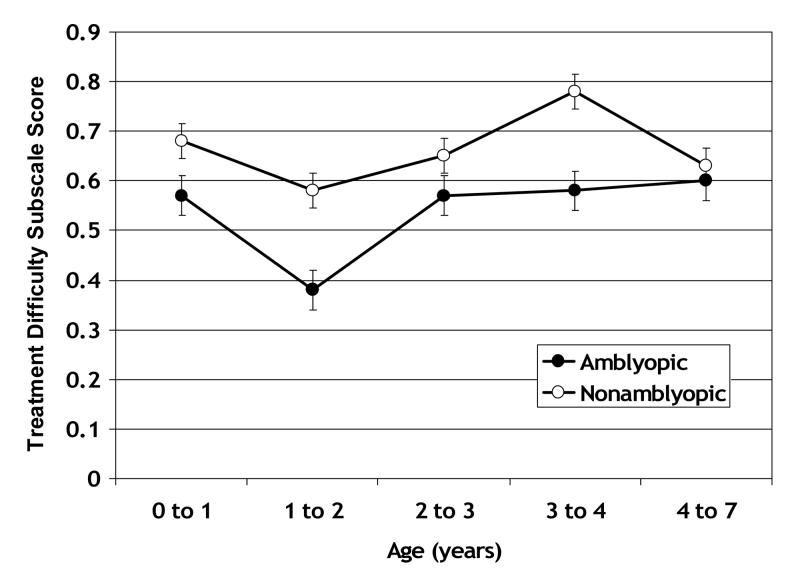
A second approach to evaluating the treatment difficulty was to look for the well-known effects of age and amblyopia on the difficulty of complying with occlusion therapy; namely, it is well known that children are more difficult to patch at ages 1-2 years and are more difficult to patch if they have amblyopia 5. Treatment Difficulty subscale scores for 78 patients who were being treated with occlusion therapy at the time of the visit at which the questionnaire was completed are summarized in Figure 4. Some patients contributed data on more than one visit and, therefore, are represented in more than one age group. Patients undergoing occlusion therapy were grouped according to whether they were being treated for amblyopia and had an interocular acuity difference of ≥0.25 logMAR or not (some patients with an interocular acuity difference ≤0.15 logMAR were occluded to wean from amblyopia treatment or alternately occluded to prevent amblyopia). A two-way ANOVA identified a significant main effect of age group (p=0.004) and a significant main effect of amblyopia (p<0.001). The interaction between age and amblyopia was not statistically significant. Overall, amblyopic patients had poorer Treatment Difficulty subscale scores. Within the amblyopic group, Treatment Difficulty subscale scores were especially poor at age 1 to 2 years (1to 2 years was significantly poorer than 0 to 1 year and 2 to 3 years by post-hoc Scheffé tests; p<0.04 for both pairwise comparisons).
Figure 4.
Treatment Difficulty subscale scores for patients who were being treated with occlusion therapy at the time of the visit on which the CVFQ was completed. Patients are grouped by whether they were being treated for amblyopia or not (some patients with equal acuity in both eyes were occluded to wean from amblyopia treatment or to prevent amblyopia). Some patients are represented in more than one age group. The number of patients contributing to each data point (mean and standard error) ranges from 9 to 39.
Alternative Treatments
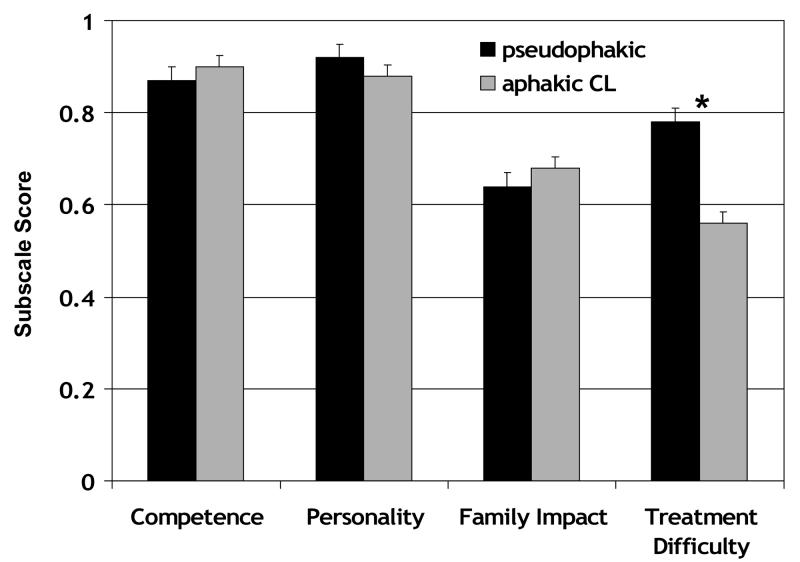
CVFQ subscale scores for 61 patients with visually significant cataracts who were optically corrected with IOL(s) or aphakic contact lens(es) are summarized in Figure 5. There was no significant difference in the mean age of the IOL and aphakic contact lens groups (p=0.82); the mean (±SD) of the IOL group was 2.9±1.9 years and of the aphakic contact lens group was 2.3±2.0 years. There was no significant difference in the mean visual acuity of the IOL and aphakic contact lens groups (p=0.15). The mean (±SD) of the IOL group was 0.58±0.35 logMAR and of the aphakic contact lens group was 0.73±0.42 logMAR. Children treated for visually significant cataract(s) with IOLs or aphakic contact lenses did not differ significantly on Competence, Personality, or Family Impact subscale scores (p>0.14 for all pairwise comparisons). Children treated for visually significant cataract(s) with aphakic contact lenses had significantly poorer Treatment Difficulty subscale scores than children treated with IOLs (p<0.001).
Figure 5.
CVFQ subscale scores (mean ± standard error) for pediatric patients treated for visually significant unilateral cataracts and were optically corrected using an IOL (pseudophakic; n=24) or an aphakic contact lens (aphakic CL; n=37). Asterisks indicate pairwise comparisons that were significant on post-hoc Scheffé tests.
Test-Retest Reliability of Subscale Scores
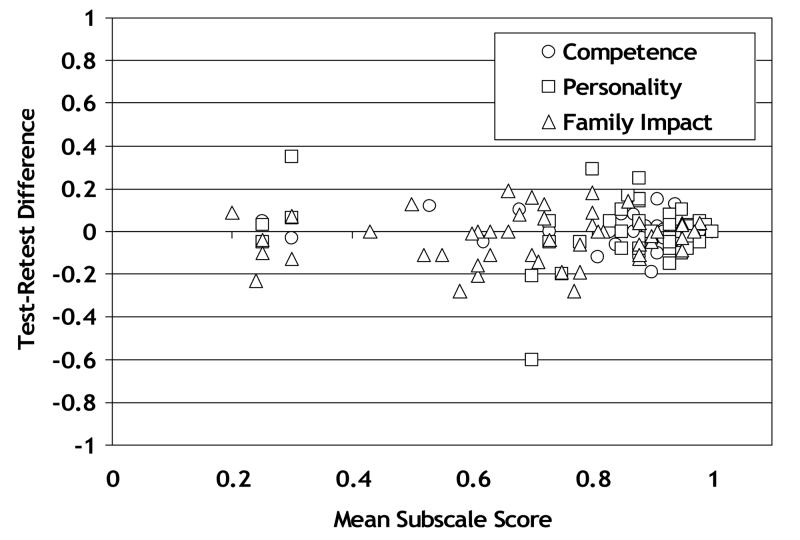
Test-retest reliability data for each subscale is shown in Figure 6. Mean differences between test and retest for the 3 subscales ranged from −0.03 to 0.00, with standard deviations ranging from 0.06 for the Competence subscale to 0.13 for the Personality subscale.
Figure 6.
Test-retest reliability for three subscale s of the CVFQ. The difference in subscale score on two questionnaires administered with 6 months (with no change in visual acuity category in either eye) is plotted as a function of the mean subscale score for both questionnaires.
Sensitivity to Changes in Treatment
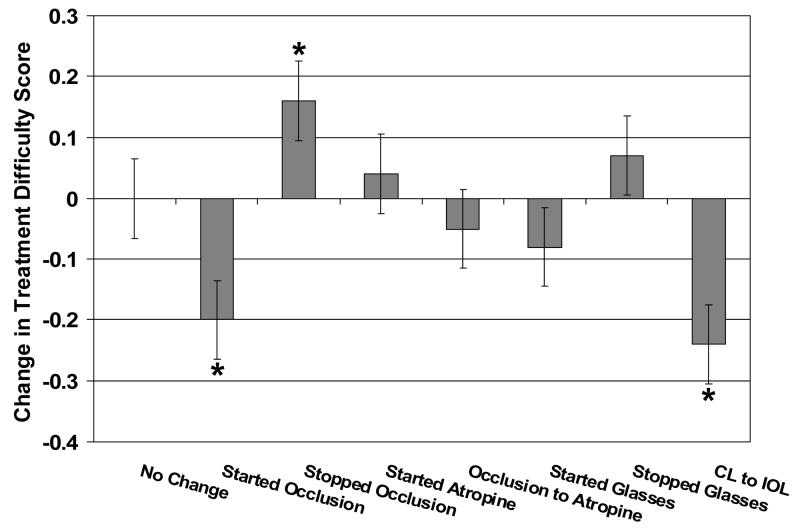
Changes in Treatment Difficulty subscale scale scores for several groups of patients whose parents completed questionnaires on two visits are summarized in Figure 7. Patients with no change, a switch from occlusion to atropine, initiation of atropine, initiation of glasses wear, and discontinuation of glasses wear had near-zero difference scores (little change in the Treatment Difficulty subscale score on the two visits; p>0.23 for all pre- post-change comparisons). Patients who started occlusion therapy had negative Treatment Difficulty subscale difference scores; i.e., increased treatment difficulty after initiation of occlusion therapy(p<0.001). Patients who stopped patching had positive Treatment Difficulty subscale difference scores; i.e., less treatment difficulty after occlusion therapy ended (p=0.045). Patients who had a secondary IOL implantation had negative Treatment Difficulty subscale difference scores; i.e., consistent with an increase in treatment difficulty (p=0.003).
Figure 7.
Changes in Treatment Difficulty subscale scores for patients whose parents completed the questionnaire on two occasions, including children who had no change in treatment between the two tests (n=49), those who started occlusion therapy after the first questionnaire (n=16), those who stopped occlusion therapy after the first questionnaire (n=22), those who started atropine penalization after the first questionnaire (n=6), those who switched from occlusion therapy to atropine penalization after the first questionnaire (n=6), those who started glasses wear after the first questionnaire (n=19), those who stopped glasses wear after the first questionnaire (n=8), and those who switched from an aphakic contact lens to an IOL after the first questionnaire (n=8). Increased treatment difficulty is downward and less treatment difficulty is upward. Asterisks indicate differences scores that are significantly different from zero.
Conclusions
The CVFQ subscales quantified meaningful differences among pediatric patient groups who were chosen to address key research questions commonly posed in ophthalmic clinical trials. As expected among the patients treated for cataracts, patients with bilateral visual impairment were reported to have significantly poorer competence than those with unilateral visual impairment while those with unilateral visual impairment reported significantly greater family impact and treatment difficulty, reflecting the more intensive occlusion treatment regimen required for visual rehabilitation in unilateral cases. Among patients with ROP, poorer visual acuity at age 3 to 7 years was associated with poorer competence, more difficult personality, and greater family impact. In addition, in a mixed group of patients being treated for anisometropia, esotropia, or cataracts, the difficulty of treatment regimen, both in terms of the type of treatment applied (spectacles, surgery, occlusion therapy, or combinations) and in terms of the age at which treatment was applied, was clearly reflected in Treatment Difficulty subscale scores. Even within the single diagnostic category of unilateral cataracts, reported treatment difficulty was significantly different for alternative treatments, with IOLs associated with less treatment difficulty than aphakic contact lenses.
Test-retest data were collected from patients who had two questionnaires completed within 6 months without a change in visual acuity category. A 4-6 month duration is typical of pediatric clinical trials 6-9, so the subscale reliability data reported here may be useful for pediatric clinical trial planning.
Preliminary data from children who were evaluated on more than one visit suggest that the Treatment Difficulty subscale is also sensitive to at least some changes in treatment regimen. Starting occlusion therapy was reported to significantly increase treatment difficulty while stopping occlusion therapy was reported to significantly reduce treatment difficulty. Less straightforward was the finding that patients with a unilateral cataract who had a secondary IOL implantation following use of an aphakic contact lens reported more treatment difficulty after IOL implantation. This was unexpected since, in a direct comparison of all patients with IOLs versus those with aphakic contact lenses, we found that IOLs were associated with less treatment difficulty. It is possible that the subgroup of patients with unilateral cataracts who received IOLs as a secondary mode of optical correction were candidates for IOLs because treatment difficulty was increasing both for contact lens use and occlusion therapy and, while the IOL reduces the treatment difficulty associated with optical correction, it does not ameliorate the treatment difficulty of occlusion therapy.
There are a number of other vision-related quality of life instruments. The Visual Function Questionnaire developed by the National Eye Institute (NEI-VFQ) is the most commonly used quality of life instrument for adults with eye disease 10-15 but several others have also been used for the assessment of vision-related quality of life in adults (reviewed by Massof and Rubin 16 and Margolis et al. 17). These instruments, including the NEI-VFQ, are generally not suitable children because many of the items assess activities which are not relevant for younger children (e.g., reading, shopping, and driving). In addition, since adults typically live independently, even if visually impaired, these instruments were not designed to assess the impact of a patient's visual impairment on other family members. Existing quality of life instruments for children 18-19 generally do not focus specifically on vision-related problems.
The finding in the present study that CVFQ subscale scores were consistent with most differences expected among well-defined patient groups supports the usefulness of the CVFQ as an outcome measure for clinical research. Incorporating the CVFQ into pediatric clinical eye studies will contribute to a more comprehensive understanding of treatment outcomes, not only in terms of potential direct benefits to the patients but also to the family. Thus, we encourage researchers to use the CVFQ to study the effects of various pediatric eye diseases and interventions on the child's day-to-day functioning and the impact of the disease and treatment regimen on the family.
The CVFQ and instructions are available free of charge on our website at http://www.retinafoundation.org/questionnaire.htm
Acknowledgments
Supported by NIH grant EY05236
Footnotes
The authors have no financial interest in the CVFQ instrument.
The study was conducted at the Retina Foundation of the Southwest, Dallas, TX
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Felius J, Stager D, Sr., Berry P, et al. Development of an instrument to assess vision-related quality of life in young children. Am J Ophthalmol. 2004;138:362–72. doi: 10.1016/j.ajo.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 3.Salomao SR, Ventura DF. Large sample population age norms for visual acuities obtained with Vistech-Teller Acuity Cards. Invest. Ophthalmol. Vis. Sci. 1995;36:657–70. [PubMed] [Google Scholar]
- 4.Moke P, Turpin A, Beck R, et al. Computerized method of visual acuity testing: adaptation of the Amblyopia Treatment Study visual acuity testing protocol for children. Am J Ophthalmol. 2001;132:903–9. doi: 10.1016/s0002-9394(01)01256-9. [DOI] [PubMed] [Google Scholar]
- 5.Pediatric Eye Disease Investigator Group A randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol. 2002;120:268–78. doi: 10.1001/archopht.120.3.268. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Ophthalmology . Pediatric Ophthalmology and Strabismus, 2002-2003. American Academy of Opthhalmology; San Francisco, CA: 2002. Basic Science and Clinical Course. Section 6; pp. 70–2. [Google Scholar]
- 7.Pediatric Eye Disease Investigator Group A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch Ophthalmol. 2003;121:603–11. doi: 10.1001/archopht.121.5.603. [DOI] [PubMed] [Google Scholar]
- 8.Pediatric Eye Disease Investigator Group A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology. 2003;110:2075–87. doi: 10.1016/j.ophtha.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Pediatric Eye Disease Investigator Group A randomized trial of atropine regimens for the treatment of moderate amblyopia in children. Ophthalmology. 2004;111:2076–85. doi: 10.1016/j.ophtha.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 10.Globe D, Varma R, Azen S, et al. Psychometric performance of the NEI VFQ-25 in visually normal Latinos: the Los Angeles Latino Eye Study. Invest Ophalmol Vis Sci. 2003;44:1470–8. doi: 10.1167/iovs.02-0292. [DOI] [PubMed] [Google Scholar]
- 11.Clemons T, Chew E, Bressler S, McBee W. National Eye Institute Visual Function Questionnaire in the Age-Related Eye Disease Study (AREDS) Arch Ophthalmol. 2003;121:211–7. doi: 10.1001/archopht.121.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nichols K, Mitchell G, Zadnik K. Performance and repeatability of the NEI-VFQ-25 in patients with dry eye. Cornea. 2002;21:578–83. doi: 10.1097/00003226-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Stelmack J, Stelmack T, Massoff R. Measuring low vision rehabilitation outcomes with the NEI VFQ-25. Invest Ophalmol Vis Sci. 2002;43:2859–68. [PubMed] [Google Scholar]
- 14.Mangione C, Lee P, Gutierrez P, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–8. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 15.Cole S, Beck R, Moke P, et al. The National Eye Institute Visual Function Questionnaire: experience of the ONTT. Invest Ophalmol Vis Sci. 2000;41:1017–21. [PubMed] [Google Scholar]
- 16.Massof R, Rubin G. Visual function assessment questionnaires. Surv Ophthalmol. 2001;45:531–48. doi: 10.1016/s0039-6257(01)00194-1. [DOI] [PubMed] [Google Scholar]
- 17.Margolis M, Coyne K, Kennedy-Martin T, et al. Vision specific instruments for the assessment of health-related quality of life and visual functioning: a literature review. Pharmacoeconomics. 2002;20:791–812. doi: 10.2165/00019053-200220120-00001. [DOI] [PubMed] [Google Scholar]
- 18.Varni J, Seid M, Kurtin P. PedsQL 4.0: relaibility and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Landgraf J, Maunsell E, Speechley K. Canadian-french, German, and UK versions of the Child Health Questionnaire: methodology and preliminary item scaling results. Qual Life Res. 1998;7:433–45. doi: 10.1023/a:1008810004694. [DOI] [PubMed] [Google Scholar]