Abstract
Rationale
Nicotine replacement therapies (NRT) have been evaluated to facilitate cigarette smoking reduction in smokers unwilling or unable to quit. In most of these studies, only conventional doses of NRT have been tested and higher doses may be required to result in significant reductions in smoking and in biomarkers of exposure.
Objective
To determine if higher NRT doses in conjunction with smoking are safe and may promote significant reductions in cigarette smoking and biomarkers of exposure.
Methods
A dose-ranging, withinsubject design was implemented to evaluate the effects of 15, 30 and 45 mg nicotine patch treatment on measures of safety and the extent of smoking reduction and biomarker exposure per cigarette in smokers (N = 20 completers) not immediately interested in quitting.
Results
Concurrent smoking and NRT were generally tolerated and resulted in no changes in blood pressure or heart rate. Slightly less than 10% of the study sample was not given the highest dose of NRT due to side effects. Self-reported cigarette smoking decreased with increasing doses of nicotine replacement and significant reductions were observed for total NNAL (a carcinogen biomarker) and carbon monoxide. However, even at the 45 mg dose, increased carbon monoxide and total NNAL per cigarette occurred, even though cotinine levels increased on average, 69.3% from baseline.
Conclusions
The present results suggest that the use of high dose NRT is safe, leads to significant reductions in smoking (-49%), significant but less reductions in total NNAL (-24%) and carbon monoxide (-37%) due to compensatory smoking.
Keywords: smoking, nicotine replacement, smoking reduction
1. Introduction
Among smokers, abstinence from tobacco has been the primary treatment goal. More recently, reduction in smoking has been a focus of study among smokers who report that they are either unwilling or unable to quit (Hatsukami et al., 2002). Cigarette reduction has been considered as a means to cessation or as a method to reduce toxicant exposure and therefore disease risk. Nicotine replacement therapies (NRTs) have been evaluated as a tool to facilitate reduced cigarette intake (Bolliger et al., 2000; Carpenter et al., 2004; Etter et al., 2002;Fagerstrom et al., 1997;Wennike et al., 2003). Although NRTs have led to greater reduction in smoking than placebo, the extent of reduction in number of cigarettes smoked does not necessarily correspond to the extent of reduction in exposure as assessed by biomarkers (Fagerstrom & Hughes, 2002;Hecht, Carmella et al., 2004;Hecht, Murphy et al., 2004;Hughes & Carpenter, 2005;Hurt et al., 2000). For example, Hecht and coworkers (2004b) found that smokers reduced their daily smoking by over 70% with the use of NRT, yet only reduced their levels of 4-(methylnitrosamino)-l-(3 pyridyl)-l-butanol (NNAL) and its glucoronides (NNAL Glucs) or total NNAL, a biomarker for the tobacco-specific lung carcinogen, 4-(methylnitrosamino)-l-(3-pyridyl)-l-butanone (NNK), by slightly over 30%. This greater reduction in cigarettes compared to biomarkers of exposure may be associated with compensatory smoking, which is smoking cigarettes in a manner that compensates for the reduced levels of nicotine by taking greater number of puffs, puff duration or volume, or inhalation volume or, in the case of low yield nicotine cigarettes, covering filter ventilation holes. Thus, the occurrence of compensatory smoking results in only modest reductions in exposure to toxicants, levels which may not lead to any significant reduction in disease risk.
The effective dose of NRT needed to minimize compensatory smoking has been rarely examined, and higher doses than those already tested may be required (Benowitz et al., 1998). In a rodent model, LeSage et al. (2003) showed dose-dependent decreases in nicotine self-administration with substantial decreases occurring at a dose of nicotine that simulates the arterial levels of nicotine attained during smoking. In addtion, studies in humans have observed that the greater the dose of exogenous nicotine, the lower the cigarette or nicotine intake from cigarettes (Benowitz & Jacob, 1990;Benowitz et al., 1998;Nemeth-Coslett & Henningfield, 1986). For example, Benowitz and coworkers (1998) found that in a group of smokers, not interested in quitting and housed in an inpatient experimental setting, increasing doses of transdermal nicotine (i.e., 21, 44, and 63 mg/day) led to an orderly decrease in nicotine intake from cigarettes (3%, 10% and 40% reduction, respectively).
To date, no outpatient study has been conducted to explore the safety and effects of high dose nicotine replacement on cigarette or nicotine selfadministration, which has been a criticism of the generalizability of prior studies (Scherer, 1999). Therefore, we conducted a modified replication of the study conducted by Benowitz et al. (1998), but in an outpatient setting. We conducted a dose-ranging pilot study examining the effects of 15, 30, and 45 mg nicotine patch treatment using a within-subject design in smokers not immediately interested in quitting. Because this study was primarily done to assess the safety of high doses in an outpatient setting, a dose run-up schedule and no placebo patches were used. Nonetheless, as a secondary outcome, we examined the relationship of NRT dose to smoking reduction and exposure to toxicants. We hypothesized that the use of high dose NRT to reduce smoking is safe and well-tolerated, and that increasing doses of the nicotine patch would result in decreasing rates of smoking and less exposure to toxicants from smoking and would minimize compensatory smoking behavior as measured by change in biomarker exposure per cigarette.
2. Materials and methods
2.1 Subjects
A multi-media strategy, including radio, television, flyers and newspaper advertisements, was used to recruit smokers interested in reducing but not in quitting. Individuals interested in the study telephoned our research clinic, and underwent screening to determine initial study eligibility. Subjects meeting the entrance criteria were invited to attend an orientation and informed consent meeting at the research clinic. Subjects were informed that the study would examine the effects of increasing doses of nicotine patch on: (a) safety; (b) number of cigarettes smoked; and (c) change in level of exposure to cigarette toxicants from change in smoking. Subjects completed a battery of self-report (i.e., tobacco use history) and physical health assessments. Subjects were invited to participate if they: (a) were between the ages of 18 and 70; (b) smoked 20-25 cigarettes/day for the past year; (c) were interested in reducing cigarette use but had no plans to set a quit date within the next 2 months; (d) were in good or stable physical health, with no history of cardiovascular disease; and (e) were in good or stable mental health. Subjects were excluded for: (a) specific medical conditions (e.g., recent myocardial infarction, serious arrhythmias, serious angina pectoris); (b) medication use that might affect tobacco use or be affected by reduction of tobacco use; (c) other tobacco or nicotine product use; or (d) were pregnant or nursing. Subjects agreed not to use nicotine products other than those assigned to them during the study and were asked not to switch brands of cigarettes. Participants were paid for involvement in the current study approved by the University of Minnesota Institutional Review Board.
2.2 Procedures
Subjects participated in the study for 12 weeks, involving three phases: (a) baseline (2 weeks), (b) treatment (5 weeks), and (c) follow-up (5 weeks after end of treatment; Figure 1).
Figure 1.
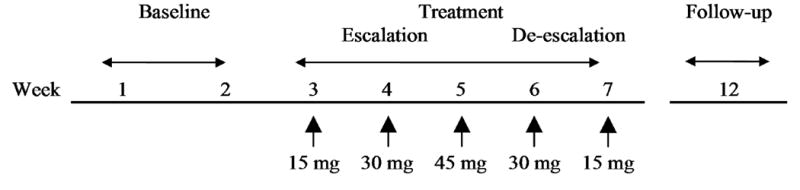
Study design and timeline. The study occurred in three phases: baseline, treatment (dose-escalation and escalation), and follow-up. For analytic purposes, only the baseline and treatment phases were evaluated.
During the baseline and treatment phases, subjects attended seven weekly visits which were consistent for day of the week and time of day within each subject. The sessions were held in the afternoon or early evening, with the exception of one patient who had morning sessions. The baseline phase (weeks 1-2) involved an orientation and then a week of data collection to evaluate baseline smoking patterns. Subsequently, participants began the five-week treatment phase that involved weekly dose changes with a dose escalation period (i.e., week 3, 15 mg; week 4, 30 mg; week 5, 45 mg) followed by dose de-escalation period (i.e., week 6, 30 mg; and week 7, 15 mg). Participants were informed of the doses that they were receiving. During the treatment phase, subjects were instructed to smoke as many cigarettes as they desired. The last two doses (i.e., week 6, 30 mg and week 7, 15 mg) were given as dose run-down primarily to minimize any potential withdrawal effects from high dose patches. However, the data during these time periods also served to determine behaviors during the descending dose limb and order effects. At each weekly treatment visit, enough nicotine patches (16-hour Nicotrol®) were dispensed to last one week and subjects were required to return all used and unused patches to determine adherence to the dosing regimen. Follow-up was conducted five weeks after end of treatment to assess health status of subjects and not for data collection purposes. For the 15 and 30 mg conditions, patches were applied in the morning and taken off at night. For the 45 mg condition, two 15 mg patches were applied in the morning and another 15 mg patch applied four hours later to minimize adverse effects (Benowitz et al., 1998). Subjects were paid $10 vouchers for each visit during the treatment period. Vouchers were redeemed at the end of treatment for payment. Subjects were paid $25 for the follow-up visit.
2.3 Measures
Unless otherwise indicated, at each clinic visit, smoking rate, subjective and physiological processes, and tobacco biomarkers were assessed.
2.3.1 Smoking
Daily cigarette smoking rate was determined by averaging self-recorded smoking captured on daily diary cards. Smokers were asked to indicate the number of cigarettes they smoked on their diary cards by using a hatch mark for every cigarette they smoked. These cards were also used to obtain information about patch use.
2.3.2 Subjective
Adverse events relating to nicotine toxicity and the nicotine patch were assessed with a researcher-administered questionnaire (Hatsukami et al., 1997;Hatsukami et al., 2003). Symptoms were rated as absent, mild, moderate or severe. Nicotine withdrawal and craving were assessed using the Minnesota Nicotine Withdrawal Scale (MNWS, Hughes & Hatsukami, 1986;Hughes & Hatsukami, 1998). Nicotine dependence was assessed using the Fagerstrom Test of Nicotine Dependence (FTND, Heatherton et al., 1991).
2.3.3 Physiological
Body weight, resting blood pressure, and heart rate were evaluated. White blood cell counts, a measure found to change with decreasing cigarette consumption (Hatsukami et al., 2005), were assessed at baseline and weeks 5 (45 mg) and 7 (15 mg).
2.3.4 Tobacco biomarkers
Expired carbon monoxide (CO) levels were evaluated at each clinic visit using a Bedfont Micro Smokerlyzer® measurement device. At baseline and weeks 5 (45 mg) and 7 (15 mg), urine samples were collected to assess for cotinine. At baseline and week 5 (45 mg), urine samples were collected to assess for total NNAL. The half-lives of these measures are about 2-4 hours for CO during waking hours (SRNT Subcommittee on Biochemical Verification, 2002), about 16 hours for cotinine (SRNT Subcommittee on Biochemical Verification, 2002), and 40 – 45 days for total NNAL (Hecht et al., 1999).
2.3.5 Exposure per cigarette ratio
Since NRT was used in this study, changes in smoke exposure per cigarette could not be directly assessed using cotinine, therefore, changes in exposure to CO and total NNAL levels served as a proxy for smoke exposure (Joseph et al., 2005;Murphy et al., 2004). In order to evaluate changes in exposure per cigarette from baseline to maximum patch dose, which may serve as a biological indicator suggestive of compensatory smoking, we employed an exposure ratio (ER):
In this formula we denote a marker of smoking exposure (e.g., NNAL) as M and daily cigarette consumption (cigarettes/day) as C. Subscripts indicate whether measures were taken at baseline (i.e., prior to treatment), or during the treatment phase. The ER is the quotient of biomarker exposure per cigarette at maximum patch dose (i.e., MT/CT) and at baseline ad libitum smoking (MB/CB). The ER can be understood as follows: (a) ER > 1, exposure per cigarette higher during treatment, suggesting compensation for nicotine; (b) ER ≅ 1, no change in exposure per cigarette; and (c) ER < 1, exposure per cigarette lower during treatment.
2.4 Analysis
Analyses were conducted with the Statistical Analysis System Version 9.1 (SAS Institute Inc., 2004). Unless otherwise stated, values of p < .05 were considered statistically significant, based on two-tailed tests. Analyses of outcome measures were restricted to the 20 subjects who had not quit smoking and who received the full course of treatment. ANOVA models were constructed that evaluated dependent measures at baseline and during the treatment phase. Dual baseline observations were averaged and used to reflect pre-treatment status on variables. Planned comparisons were made between each dose in the treatment phase and baseline as well as between consecutive doses during the escalation and de-escalation periods. Observations made at follow-up were not included since these were collected for safety-monitoring purposes. Paired-sample t-tests were used for some comparisons.
3. Results
3.1 Sample Characteristics
Of the 64 interested participants who signed consent forms at the orientation, 22 completed the baseline assessment forms (2 did not) only to drop from the study after this point. Forty subjects attended the physical examination, of whom 10 were excluded from participation. Thirty subjects returned for the final baseline session, with 25 subjects remaining in the study through the treatment period. Three subjects quit smoking during the treatment period. Two subjects developed side effects (e.g., lightheadness and marked irritability) and did not complete 45 mg patch therapy. Twenty subjects continued to smoke during the treatment phase, and received the full course of nicotine replacement.
In the restricted sample of 20 participants who completed treatment and were smoking, 65% were male. The sample had a mean age of 40.8 years (SD = 9.8) with most (70%) having some college education. The sample had the following smoking characteristics: cigarettes/day, M = 26.3, SD = 6.1; carbon monoxide (ppm), M = 23.9, SD = 7.9; years of smoking at current rate, M = 16.7, SD = 10.1; number of quit attempts, Median = 3.0; FTND score, M = 6.1, SD = 2.1; and cigarette filter type, regular/medium, 20%, lights/ultralights, 80%. Comparison of the 20 completers to the 42 subjects evaluated for baseline characteristics who did not complete treatment revealed no significant differences on any of the foregoing variables.
3.2 Compliance
The mean rates of self-reported patch use out of the total required number of patches per week were as follows: Week 3, 15 mg, (90.0%, n = 20); Week 4, 15 mg, (87.1%, n = 20); Week 5, 45 mg, (91.3%, n = 18); Week 6, 30 mg, (91.4%, n = 20); and Week 7, 15 mg, (91.7%, n = 19). Rates of adherence did not differ across the treatment period, χ2(4) = 3.91, 0.4186. On average, 71% of used patches were brought back (patients frequently reported inadvertently discarding used patches).
3.3 Safety and Side Effects
As noted previously, 2 of the 25 subjects were not able to tolerate the 45 mg patch. The rates of the most common nicotine-patch associated side effects were computed and are displayed in Table 1.
Table 1.
Endorsement Percentages of 8 Common Nicotine Patch-Related Side Effects
| Baseline | Treatment | |||||
|---|---|---|---|---|---|---|
| Side Effect | 15 mg | 30 mg | 45 mg | 30 mg | 15 mg | |
|
|
||||||
| Dizziness | 5 | 0 | 5 | 35 | 10 | 0 |
| Lightheadedness | 0 | 5 | 15 | 20 | 5 | 0 |
| Nausea | 10 | 15 | 25 | 35 | 15 | 15 |
| Sleep disturbance | 5 | 5 | 0 | 5 | 0 | 10 |
| Muscle Pain | 0 | 5 | 0 | 0 | 0 | 0 |
| Swellinga | 0 | 15 | 10 | 5 | 0 | 10 |
| Rednessa | 0 | 35 | 30 | 30 | 15 | 0 |
| Itchinga | 5 | 0 | 5 | 35 | 10 | 0 |
Note. N = 20. Percentages indicate those endorsing any level of the side effect, mild, moderate or severe.
Indicates swelling, redness, or itching at the patch site.
Some side effects showed systematic, dose-related changes, including sleep disturbance, dizziness, lightheadedness, nausea and itching.
3.3.1 Vitals
Systolic and diastolic blood pressure, heart rate, and weight did not vary between the baseline and treatment phases (all ps > .25)
3.4 Smoking and Smoke Exposure
3.4.1 Cigarettes/day
The effects of nicotine patch dose on cigarettes/day from baseline through the treatment phase were evaluated (Table 2).
Table 2.
Mean and Percentage of Baseline Levels Across Doses for Outcome Measures
| Baseline | Treatment | |||||
|---|---|---|---|---|---|---|
| 15 mg | 30 mg | 45 mg | 30 mg | 15 mg | ||
|
|
||||||
| Cigarettes/day | ||||||
| Mean (SD) | 26.6 (9.3) | 20.3 (9.1)* † | 16.3 (8.3)* † | 13.6 (8.3)* | 15.8 (10.5)* | 16.4 (10.5)* |
| % Baseline | – | 76.3 | 61.3 | 51.1 | 59.4 | 61.7 |
| CO (ppm) | ||||||
| Mean (SD) | 25.0 (7.9) | 21.4 (9.3)* † | 18.6 (10.2)* † | 15.7 (6.4)* | 17.0 (11.0)* | 16.5 (7.0)* |
| % Baseline | – | 85.6 | 74.4 | 62.8 | 68.0 | 66.0 |
| Cotinineabc | ||||||
| Mean (SD) | 7,170 (3,120) | – | – | 12,140 (5,690)* | – | 9,740 (6,070) |
| % Baseline | – | – | – | 169.3 | – | 135.8 |
| Total NNALabd | ||||||
| Mean (SD) | 2.50 (1.04) | – | – | 1.91 (.90)* | – | – |
| % Baseline | – | – | – | 76.1 | – | – |
Note. N = 20.
Quantities adjusted for urine concentration by dividing by creatinine level (mg).
Quantities not assessed at 15 and 30 mg time points.
(ng/mL).
(pmol/mL)
Significant differences between consecutive time points are shown with a dagger (†) while significant differences between treatment time points and baseline were shown with an asterisk (*).
Cigarette smoking declined steadily during dose escalation phase before stabilizing in the dose de-escalation phase, linear, F(1, 106) = 28.64, p <.0001, quadratic, F(1, 101) = 19.22, p <.0001. Daily smoking at each week of patch treatment was significantly lower than baseline (all ps <.01). During the dose escalation phase, significant differences between consecutive weeks of treatment were observed between 15 mg (week 3) and 30 mg (week 4), t(116) = −5.81, p <.0001; 30 mg (week 4) and 45 mg (week 5), t(103) = −3.81, p = .0002. No differences in daily smoking rate were seen between weeks 5 and 6 or between weeks 6 and 7.
3.4.2 Carbon monoxide
Mean carbon monoxide levels declined during the dose escalation phase before stabilizing in the dose de-escalation phase, linear, F(1, 116) = 7.29, p = .0080, quadratic, F(1, 114) = 3.66, p = .0583 (Table 2). Carbon monoxide levels at each week of patch treatment were lower than baseline (all ps <.01). Significant differences between consecutive weeks of treatment were observed between 15 mg (week 3) and 30 mg (week 4), t(112) = −3.36, p = .0004; and 30 mg (week 4) and 45 mg (week 5), t(87) = −3.25, p = .0016. No differences in carbon monoxide level were seen between weeks 5 and 6 or between weeks 6 and 7.
3.4.3 Cotinine levels
Log-normalized urine cotinine levels were compared at baseline, and at two treatment time points when cotinine was also assayed (week 5, 45 mg; week 7, 15 mg). Cotinine levels varied significantly over the observation period, linear, F(1, 40.6) = 9.42, p = .0038, quadratic, F(1, 39.1) = 8.60, p = .0056 (Table 2 for mean values). Cotinine levels rose sharply from baseline to the 45 mg (week 5) observation, t(42.3) = 3.21, p = .0025, but returned to baseline levels after the last week at the 15 mg dose (week 7).
3.4.4 Total NNAL
Total NNAL was measured at baseline and at the maximum dose of NRT at week 5 (45 mg). Using a paired-sample t-test, log-normalized, absolute NNAL levels declined significantly, t(19) = -3.48, p = 0.0025 (Table 2).
3.5 Exposure per cigarette ratio
Exposure per cigarette ratios were used to index changes in two toxicants, CO and NNAL, from baseline to the highest dose week (week 5, 45 mg) when maximal smoking reduction was expected and observed. CO ERs were also evaluated throughout the 5-week treatment period. Increases in ERs indicated increased inhalation of mainstream smoke, and probable compensation for nicotine. Figure 2 shows the distribution of ERs across individuals at the 45 mg nicotine patch dose in relation to percentage reduction in smoking (cigarettes/day). This figure demonstrates individual variability in response to NRT. Some individuals showed a dramatic reduction in cigarette intake and others showed a minimal response to high doses of nicotine patch. Similar variability across subjects was observed for exposure ratios.
Figure 2.
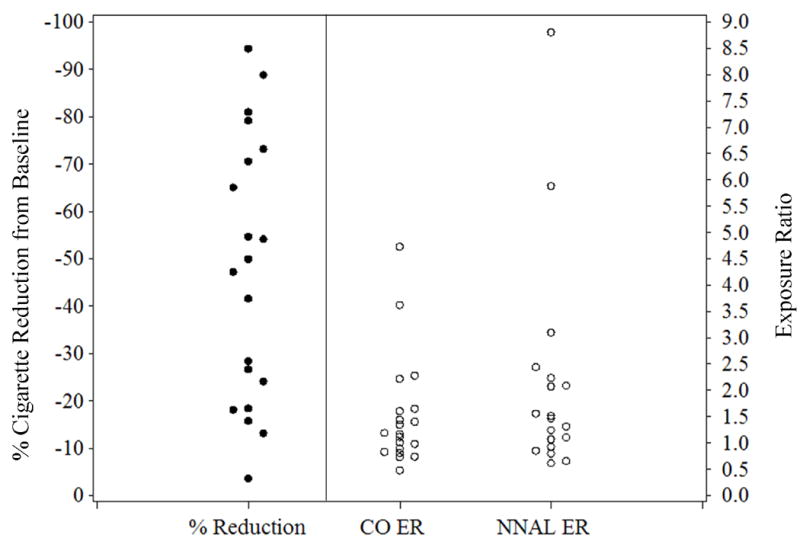
Percentage reduction in smoking at week 5 (45 mg dose) and exposure ratios for carbon monoxide and total NNAL at week 5 across subjects. Mean reduction at week 5 was -48.9%, while mean ERs for carbon monoxide and total NNAL at week 5 were respectively, M = 2.45 (SD = 2.54) and M = 1.66 (SD = 1.09).
3.5.1 Carbon monoxide
ERs remained stable across the treatment period, linear, F(1, 82.5) = 0.63, p = n.s., quadratic, F(1, 79.8) = 0.32, p = n.s. Mean ERs for each week of patch treatment were: Week 3 (15 mg, M = 1.26, SD = 0.61), Week 4 (30 mg, M = 1.33, SD = 0.68), Week 5 (45 mg, M = 1.66, SD = 1.09), Week 6 (30 mg, M = 1.30, SD = 0.69), and Week 7 (15 mg, M = 1.49, SD = 1.14). Exposure per cigarette was significantly elevated from baseline at Week 4, t(31.6) = 2.27, p = .0301; Week 5, t(37.0) = 2.63, p = .0125; Week 6, t(31.8) = 2.98, p = .0055; and Week 7, t(53.4) = 2.49, p = .0157.
3.5.2 Total NNAL
Increases in total NNAL per cigarette were seen, with a significant increase in creatinine-adjusted total NNAL per cigarette, suggesting compensatory smoking (M = 2.45 ER, SD = 2.54), t(19) = 2.56, p = .0192.
3.5.3 Percentage reduction and ER
Greater reduction in cigarettes was associated with greater exposure per cigarette, both CO ER, r(18) = .72, p = .0004, and total NNAL ER, r(18) = .74, p = .0002. Moreover the CO ER and total NNAL ERs were strongly associated, r(18) = .87, p < .0001.
3.6 Physiological Processes
3.6.1 White blood cell count
White blood cell count was measured at baseline and weeks 5 (45 mg) and 7 (15 mg). White blood cell count did not change significantly from baseline through the treatment period, linear, F(1, 22.8) = 0.46, n.s., quadratic, F(1, 22.4) = 0.63, n.s.
3.7 Nicotine Craving and Withdrawal
Self-reported nicotine withdrawal on the Minnesota Nicotine Withdrawal Scale, including the 7 DSM-IV items and not craving, did not vary significantly from baseline through the treatment phase, linear, F(1, 112) = 1.01, n.s., quadratic, F(1, 109) = .43, n.s. However, analysis of the craving item of the MNWS showed both linear, F(1, 113) = 11.90, p = .0008, and quadratic, F(1, 110) = 9.48, p = .0026 effects. Craving declined significantly from baseline to week 3 (15 mg), t(116) = -3.55, p = .0006, and from week 3 (15 mg) to week 4 (30 mg), t(115) = -3.42, p = .0009. After week 4, craving remained relatively stable.
4. Discussion
This pilot study showed that using high doses of NRT in healthy pack-a-day or more smokers produced increasing side effects with increasing nicotine dose, however, they were generally tolerated, although 2 out of 25 subjects were excluded from using the 45 mg patch due to side effects. High dose NRT appeared to have little effect on physiological processes such heart rate, blood pressure, or weight, results that are concordant with an inpatient study (Zevin et al., 1998). Furthermore, significant dose-related reductions in daily cigarette consumption can be achieved in an outpatient setting through high-dose nicotine therapy. Levels of reduction in cigarettes and CO were sustained even during the descending dose limb. Given the use of nicotine replacement, it is not surprising that reduction in daily smoking did not result in nicotine withdrawal and craving reduced significantly over time.
Although significant reductions were observed for number of cigarettes smoked and this reduction was dose-related, the extent of reduction at the highest dose was not as high as expected. The mean percentage cigarette reduction with the 45 mg patch was 49%, even though the levels of cotinine increased by 69%. Nevertheless, this level of reduction was greater than that seen during an inpatient study in which smokers received doses ranging from placebo to 63 mg (3, 21 mg, 24-hour patches) for a period of 5 days. Benowitz et al., (1998) saw a maximum of a 26% reduction in cigarettes at the highest dose as compared to placebo patch. This modest reduction was observed in the face of a 250% increase in plasma nicotine levels. The lower level of reduction observed in their study was attributed to the study’s inpatient setting, with its limited exposure to smoking cues that typically occur in the subject’s natural environment, leading to lower rates of smoking across all conditions including placebo. In fact, the authors noted that overall smoking on the unit was diminished, with pre-clinic ad libitum smoking of 29 cigarettes/day compared to just 17 cigarettes/day while the smokers were on placebo patch on the unit. Similar observations were made in another inpatient study in which smokers were assigned to 0, 22 and 44 mg 24-hour patches in randomized, counterbalanced order, with each patch dose worn for a period of 1 week (Pickworth et al., 1994). In the higher dose condition, subjects attained a 215% increase in nicotine concentration and only a 21% decrease in cigarettes compared to the placebo condition. Thus, very high doses of nicotine delivered transdermally do not lead to dramatic reductions in cigarettes smoked, particularly among smokers not interested in quitting or, as in the case of the two inpatient studies, in reducing.
This study also showed that absolute reductions in both carbon monoxide and total NNAL were not proportional to reductions in cigarettes/day, as has been observed in previous studies (e.g., Fagerstrom & Hughes, 2002;Hecht, Murphy et al., 2004;Hughes & Carpenter, 2005). In a review conducted by Hughes and Carpenter (2005), studies on cigarette reduction interventions for smokers not trying to quit were examined. Of the studies that used typical doses of NRT and that described both reductions in cigarettes per day and CO, the respective mean reductions were 39% (range = 18%-66%) and 27% (range= 10% to 46%). In the review by Fagerstrom and Hughes (2002), studies using usual doses of gum, inhaler or patches with instructions or intentions to reduce, the mean reduction in cigarettes was about 50% and the mean reduction in CO was about 30%. In nicotine patch studies that involved instructions to smoke cigarettes ad libitum, the mean cigarette reduction was about 40% and the mean CO reduction was about 30%. We observed similar reductions in cigarettes (49%) and CO (37%) with the 45 mg patch. With carbon monoxide’s short half-life, reductions should have been immediately apparent at any of the doses; on the other hand, the time of last cigarette may have a significant influence on this biomarker of exposure. Total NNAL is a better biomarker of exposure because of its longer half-life, however, the modest changes in levels of total NNAL (24%) relative to the changes observed for CO (37%) must be interpreted cautiously because of its elimination half-life of approximately 40 – 45 days (Hecht et al., 1999). Therefore, lower total NNAL levels may be observed with longer duration on the patch. It is unknown whether or not the reductions in carbon monoxide and total NNAL observed in this study would reduce disease risk, however even these modest reductions may have a significant population impact.
Apart from the question of absolute reductions in smoking and biomarkers of exposure is the issue of compensatory smoking for nicotine and relative exposure to toxicants, such as CO and NNAL, on a per cigarette basis. Benowitz et al. (1998)reported that the average nicotine intake from cigarette smoking was observed to be reduced by 40% on the highest dose patch (i.e., 63 mg). This finding suggests that there was a general decreased inhalation of mainstream smoke. Furthermore, based on our calculations of the data presented in the Benowitz paper, the ER for area under the curve blood carboxyhemoglobin was 0.976 at the 63 mg patch, which would indicate no compensation. Although nicotine levels were not measured in our study, we did measure tobacco toxicants associated with tobacco intake. Unlike the results from the Benowitz et al., (1998)study, we observed that ER for CO and NNAL both increased substantially. CO exposure per cigarette increased by a factor of 1.66 and total NNAL increased by a factor of 2.45. The reason for the discrepancy in results between these two studies is unknown except perhaps again the differences in the settings of each of these studies – an inpatient where there are few cues that are normally associated with smoking versus outpatient in which smokers are in their natural environment.
We found a relationship between the amount of cigarette reduction and the extent of exposure per cigarette, such that, the greater the reduction in smoking, the greater the extent of exposure per cigarette, even with increasing levels of cotinine resulting from the assignment to higher doses of transdermal nicotine. Several reasons may account for this counterintuitive finding. Higher doses of nicotine may result in greater desensitization of brain nicotinic cholinergic receptors and therefore less reinforcement from nicotine (Benowitz et al., 1998). This blunted reinforcement or tolerance may lead to greater inhalation per cigarette. Alternatively, nicotine replacement that is delivered transdermally may not result in smoke exposure reduction that may be observed if nicotine was administered in a bolus fashion as found with cigarettes. The smoker may be compensating for the lack of rapid delivery of nicotine to the brain. Perhaps a combination of high dose nicotine plus an ad libitum NRT, such as nicotine nasal spray, may have resulted in greater reductions in cigarettes and less smoke exposure per cigarette. It is also possible that with decreasing cigarettes, smokers are compensating for constituents other than nicotine (e.g., MAO inhibitors, Fowler et al., 2003) or for the sensory stimulation from smoking (Rose, 2006;Rose et al., 1993).
As a final point, the mean reduction of cigarette smoking and the mean exposure ratio do not reveal the individual variability that is associated with the response to the nicotine patch. For example, a significant number of smokers showed a greater than 50% reduction in smoking. Future studies, the reasons associated with these differences (e.g., nicotine metabolism, extent of desensitization of receptors) should be investigated.
Four main limitations are associated with this study. First, we were not able to verify the number of cigarettes smoked and it is possible that subjects underreported their cigarette intake. However, there was no reason for inaccurate reporting because no smoking reduction level was required of the subjects. Second, we were not able to objectively verify the actual amount of nicotine patch use. Subjects were asked to return all used and unused patches; however, due to lost and inadvertently discarded patches, a full, objective accounting of patch use was not possible. Third, because this was a pilot study primarily designed to examine safety, we failed to use a placebo patch which would be critical in examining the actual impact of NRT on smoking behavior. Furthermore, the safety context of this study and the concern over the administration of three nicotine patches may have significantly impacted smoking rate. Finally, the blood concentrations during the assessment period for the 45 mg patch may not have been optimal because the third patch was applied at noon rather than in the morning and patches were removed during the evening to minimize potential side effects. This method of patch administration may not have led to the greatest impact on smoking behavior.
In summary, the results of this study showed that high doses of nicotine replacement are safe when used on an outpatient basis in dependent smokers. It also led to reductions in smoking and biomarkers of exposure in a sample of smokers who were interested but not required to reduce their cigarette consumption. Nonetheless, even with the attainment of high nicotine replacement levels as reflected by the increase in cotinine, a significant number of subjects appear to have increased inhalation of mainstream smoke per cigarette, as reflected by relative increases of two tobacco toxicants, CO and NNAL, per cigarette. This finding would suggest that the use of high doses of transdermal nicotine can reduce exposure to toxicants but to levels that may or may not lead to reduced health risk due to compensatory smoking.
Acknowledgments
This research was supported by the NIDA grant P50-DA13333. The second author received support from NIDA grant K01-DA019446. GlaxoSmithKline provided the nicotine patches. We wish to thank Neal Benowitz for discussions on the design of the current study and Kevin Delucchi of analytic assistance. Rachel Feuer made helpful comments on this manuscript for which we thank her. We also thank the participants for taking part in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication.As a service to our customers we are providing this early version of the manuscript.The manuscript will undergo copyediting, typesetting, and review of the resulting proofbefore it is published in its final citable form. Please note that during the productionprocess errorsmaybe discovered which could affect the content, and all legal disclaimersthat apply to the journal pertain.
References
- Benowitz NL, Jacob P., 3rd Intravenous nicotine replacement suppresses nicotine intake from cigarette smoking. J Pharmacol Exp Ther. 1990;254(3):1000–5. [PubMed] [Google Scholar]
- Benowitz NL, Zevin S, Jacob P., 3rd Suppression of nicotine intake during ad libitum cigarette smoking by high-dose transdermal nicotine. J Pharmacol Exp Ther. 1998;287(3):958–62. [PubMed] [Google Scholar]
- Bolliger CT, Zellweger JP, Danielsson T, van Biljon X, Robidou A, Westin A, et al. Smoking reduction with oral nicotine inhalers: double blind, randomised clinical trial of efficacy and safety. Bmj. 2000;321(7257):329–33. doi: 10.1136/bmj.321.7257.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol. 2004;72(3):371–81. doi: 10.1037/0022-006X.72.3.371. [DOI] [PubMed] [Google Scholar]
- Etter JF, Laszlo E, Zellweger JP, Perrot C, Perneger TV. Nicotine replacement to reduce cigarette consumption in smokers who are unwilling to quit: A randomized trial. Journal of Clinical Psychopharmacology. 2002;22(5):487–95. doi: 10.1097/00004714-200210000-00008. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Hughes JR. Nicotine concentrations with concurrent use of cigarettes and nicotine replacement: a review. Nicotine Tob Res. 2002;4 (Suppl 2):S73–9. doi: 10.1080/1462220021000032753. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Tejding R, Westin A, Lunell E. Aiding reduction of smoking with nicotine replacement medications: hope for the recalcitrant smoker? Tob Control. 1997;6(4):311–6. doi: 10.1136/tc.6.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Logan J, Wang GJ, Volkow ND. Monoamine oxidase and cigarette smoking. Neurotoxicology. 2003;24(1):75–82. doi: 10.1016/s0161-813x(02)00109-2. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Grillo M, Pentel PR, Oncken C, Bliss R. Safety of cotinine in humans: physiologic, subjective, and cognitive effects. Pharmacol Biochem Behav. 1997;57(4):643–50. doi: 10.1016/s0091-3057(97)80001-9. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Jensen J, Brauer LH, Mooney M, Schulte S, Sofuoglu M, et al. Lack of effect of 5HT(3) antagonist in mediating subjective and behavioral responses to cotinine. Pharmacol Biochem Behav. 2003;75(1):1–7. doi: 10.1016/s0091-3057(03)00035-2. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Kotlyar M, Allen S, Jensen J, Li S, Le C, et al. Effects of cigarette reduction on cardiovascular risk factors and subjective measures. Chest. 2005;128(4):2528–37. doi: 10.1378/chest.128.4.2528. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Slade J, Benowitz NL, Giovino GA, Gritz ER, Leischow S, et al. Reducing tobacco harm: research challenges and issues. Nicotine Tob Res. 2002;4 (Suppl 2):S89–101. doi: 10.1080/1462220021000032852. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Chen M, Dor Koch JF, Miller AT, Murphy SE, et al. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59(3):590–6. [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Le KA, Murphy SE, Li YS, Le C, et al. Effects of reduced cigarette smoking on levels of 1-hydroxypyrene in urine. Cancer Epidemiol Biomarkers Prev. 2004;13(5):834–42. [PubMed] [Google Scholar]
- Hecht SS, Murphy SE, Carmella SG, Zimmerman CL, Losey L, Kramarczuk I, et al. Effects of reduced cigarette smoking on the uptake of a tobacco-specific lung carcinogen. J Natl Cancer Inst. 2004;96(2):107–15. doi: 10.1093/jnci/djh016. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Carpenter MJ. The feasibility of smoking reduction: an update. Addiction. 2005;100(8):1074–89. doi: 10.1111/j.1360-0443.2005.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Errors in using tobacco withdrawal scale. Tob Control. 1998;7(1):92–3. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt RD, Croghan GA, Wolter TD, Croghan IT, Offord KP, Williams GM, et al. Does smoking reduction result in reduction of biomarkers associated with harm? A pilot study using a nicotine inhaler. Nicotine Tob Res. 2000;2(4):327–36. doi: 10.1080/713688154. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Hecht SS, Murphy SE, Carmella SG, Le CT, Zhang Y, et al. Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2963–8. doi: 10.1158/1055-9965.EPI-04-0768. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology (Berl) 2003;170(3):278–86. doi: 10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Link CA, Jensen J, Le C, Puumala SS, Hecht SS, et al. A comparison of urinary biomarkers of tobacco and carcinogen exposure in smokers. Cancer Epidemiol Biomarkers Prev. 2004;13(10):1617–23. [PubMed] [Google Scholar]
- Nemeth-Coslett R, Henningfield JE. Effects of nicotine chewing gum on cigarette smoking and subjective and physiologic effects. Clin Pharmacol Ther. 1986;39(6):625–30. doi: 10.1038/clpt.1986.110. [DOI] [PubMed] [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl) 2006;184(34):274–85. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Levin ED. Role of nicotine dose and sensory cues in the regulation of smoke intake. Pharmacol Biochem Behav. 1993;44(4):891–900. doi: 10.1016/0091-3057(93)90021-k. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc.(2004). The SAS System for Windows (Version 9.1). Cary, NC: SAS Institute Inc
- Scherer G. Smoking behaviour and compensation: a review of the literature. Psychopharmacology (Berl) 1999;145(1):1–20. doi: 10.1007/s002130051027. [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation Nicotine Tob Res (2002);4(2):149-59
- Wennike P, Danielsson T, Landfeldt B, Westin A, Tonnesen P. Smoking reduction promotes smoking cessation: results from a double blind, randomized, placebo-controlled trial of nicotine gum with 2-year follow-up. Addiction. 2003;98(10):1395–402. doi: 10.1046/j.1360-0443.2003.00489.x. [DOI] [PubMed] [Google Scholar]
- Zevin S, Jacob P, 3rd, Benowitz NL. Dose-related cardiovascular and endocrine effects of transdermal nicotine. Clin Pharmacol Ther. 1998;64(1):87–95. doi: 10.1016/S0009-9236(98)90026-1. [DOI] [PubMed] [Google Scholar]


