Abstract
Background
Opiate-dependent individuals are prone to dysphoria that may contribute to treatment failure. Methadone-maintenance therapy (MMT) may mitigate this vulnerability, but controversy surrounds its long-term use. Little is known about the neurobiology of mood dysregulation in individuals receiving or removed from MMT.
Methods
Fifteen opiate-abstinent and 12 methadone-maintained, opiate-dependent subjects, who lacked other Axis I pathology, and 13 control subjects were compared on the Cornell Dysthymia Rating Scale (CDRS) and regional cerebral glucose metabolism (rCMRglc) using [18F]fluorodeoxyglucose positron emission tomography.
Results
CDRS scores showed no group differences. Opiate-abstinent subjects had lower rCMRglc than control subjects in the bilateral perigenual anterior cingulate cortex (ACC), left mid-cingulate cortex, left insula and right superior frontal cortex. Methadone-maintained subjects exhibited lower rCMRglc than control subjects in the left insula and thalamus. In opiate-abstinent subjects, rCMRglc in the left perigenual ACC and mid-cingulate cortex correlated positively with CDRS scores.
Conclusions
In remitted heroin dependence, opiate-abstinence is associated with more widespread patterns of abnormal cortical activity than MMT. Aberrant mood processing in the left perigenual ACC and mid-cingulate cortex, seen in opiate-abstinent individuals, is absent in those receiving MMT, suggesting that methadone may improve mood regulation in this population.
Keywords: methadone, heroin, abstinence, depression, mood, positron emission tomography
1. Introduction
Opiate-dependent individuals are often depressed (Nunes et al., 2004), and suicide is fourteen times as frequent in this group as in the general population (Darke and Ross, 2002). Related to these findings are data from functional imaging studies, which indicate involvement of the central endogenous opioid system in regulation of affect. For instance, healthy subjects demonstrate a decrease in mu-opioid neurotransmission in the rostral anterior cingulate gyrus, ventral pallidum, and amygdala during induced sadness (Zubieta et al., 2003). Therefore, even in the absence of a comorbid mood disorder, opiate-dependent individuals may have lasting vulnerability to affect dysregulation through altered functioning of brain regions that are rich in mu-opioid receptors and are important in processing negative mood states.
Methadone maintenance therapy (MMT) is currently the primary treatment modality for opiate-dependence in the United States, and long-term treatment has been deemed crucial for a positive outcome (Ball and Ross, 1991; Flynn et al., 2003). Clients who receive MMT and remain in treatment show significant improvement in depressive symptoms (Rounsaville et al., 1982; Musselman and Kell, 1995; Dean et al., 2004). In contrast, during protracted abstinence from opiates, former heroin users are particularly prone to depressive states (Mason et al., 1998; Nunes et al., 2004), which may persist (Latowsky, 1996; Satel et al., 1993) and contribute to relapse (Gossop et al., 2002; Rao et al., 2004). Prior studies have not examined neural function related to depression in long-term opiate-abstinent and methadone-maintained, former heroin users.
The few studies that have investigated brain function in clients receiving MMT involved research participants who did not use illicit drugs (Rose et al., 1996; Krystal et al., 1995; Danos et al., 1998), as well as other that were actively using heroin (Pezawas et al., 2002) or were HIV-positive (Woods et al., 1991). The resultant reports yielded inconsistent findings of cortical and subcortical perfusion defects. In a preliminary study, four opiate-abstinent subjects in a community MMT program showed enhanced relative regional cerebral glucose metabolism (rCMRglc) in the perigenual anterior cingulate cortex (ACC) compared to control subjects (Galynker et al., 2000). Another study found abnormal phospholipid metabolism in opiate-dependent subjects, with some return to baseline following longer-term MMT (Kaufman et al., 1999).
Though we are unaware of imaging studies that focus on mood in opiate-dependent subjects, there is a growing literature examining the functional neurobiology of depression in research participants who do not use drugs of abuse. Some investigations revealed lower cerebral glucose metabolism and/or perfusion in the medial prefrontal cortex, including orbitofrontal and perigenual anterior cingulate regions, and in the parietal cortices of depressed subjects (see reviews by Drevets 2000, 2000b). Others showed higher activity in subgenual ACC (Mayberg et al., 1999) and alterations in function and structure of the amygdala (Drevets et al., 2002; Sheline et al., 2001), insula, and thalamus than in healthy control subjects (Delgado et al., 1999). In addition, a study of dysthymic individuals demonstrated widely distributed deficits in cortical perfusion, including effects in the bilateral inferior parietal cortex, and left superior frontal and posterior temporal cortex (Sarikaya et al., 1999).
In this study, we used [18F]fluorodeoxyglucose (FDG) and positron emission tomography (PET) to study rCMRglc in two groups of stable, opiate-dependent individuals in full, sustained remission without mood disorders or other Axis I pathology and in healthy comparison subjects. Participants in one of the groups had been treated with MMT and were in protracted opiate-abstinence at the time of the study; those in the other group were receiving long-term MMT at the time of the study. We selected eight bilateral brain regions for testing, based on the literature on depression (subgenual and perigenual ACC, dorsal cingulate gyrus, medial orbitofrontal cortex, inferior parietal cortex, insula, amygdala, and thalamus). Our hypotheses were: 1) that opiate-abstinent, heroin-dependent participants would show abnormalities in rCMRglc measured in these brain regions that have been implicated as playing a role in depression, and 2) that participants receiving MMT would exhibit less of these abnormalities.
2. Methods and Materials
2.1. Study participants
All research procedures were approved by the Institutional Review Boards of NIDA and Beth Israel Medical Center. After receiving a detailed explanation of the study, the subjects gave their written informed consent to participate. They received financial compensation following their participation. Participants were recruited to three groups: 1) opiate-dependent subjects in sustained abstinence (opiate-abstinent); 2) opiate-dependent subjects receiving MMT (methadone-maintained); and 3) control subjects (Table 1). For inclusion in the two opiate dependent groups, a DSM-IV diagnosis of opiate dependence in the 2 years before entry with 18 months of abstinence from illicit drug use was required. Opiate-abstinent subjects were recruited from the Su Casa Methadone to Abstinence Residential Program. They were required to have a history of prior MMT for >1 year and no MMT for at least 6 months prior to enrollment. Methadone-maintained subjects were recruited from the Su Casa Short Stay Program. They were required to have received MMT for >1 year and to have taken a stable methadone dose for at least 6 months before enrollment. Participants were of both sexes, 21-55 years of age, and right-handed (score >20, modified Edinburgh Handedness Scale (McKeever 1995)).
Table 1.
Characteristics of Research Participants
| Subject Group: | Control
(n = 13) |
Methadone-Maintained
(n = 12) |
Opiate-Abstinent
(n = 15) |
|---|---|---|---|
| Age (years)a | 33.15 ± 2.67 | 41.2 ± 2.01* | 42.1 ± 1.68* |
| Education (years)a | 15.2 ± 0.65 | 11.9 ± 0.56* | 11.9 ± 0.62* |
| Mother’s Education (years)b | 13.0 ± 0.99
(n = 11) |
10.6 ± 1.40
(n =7) |
7.6 ± 1.43
(n = 9) |
| Sex (percent male) | 69.2 | 83.3 | 80.0 |
| Race (percent): | |||
| Caucasian non-Hispanic | 61.5 | 41.7 | 26.7 |
| Caucasian Hispanic | 0 | 33.3 | 46.7 |
| African American | 30.8 | 25.0 | 26.7 |
| Asian | 7.7 | 0 | 0 |
Data shown are means and standard errors of the means.
Some of the subjects were adopted or did not know their mother’s educational level.
Significantly different from control group, p<0.05 by Student’s t-test.
Exclusion criteria were current or lifetime history of any Axis I diagnosis other than opiate dependence (formerly opiate-abusing groups) or nicotine dependence (all groups) as assessed by the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1996). Furthermore, to exclude subjects who were acutely depressed during testing, we administered the Hamilton Rating Scale for Depression (HRSD) (Hamilton 1960), using an exclusion criterion of a score >12. The groups did not differ on the HRSD (mean±SEM): 8.38±1.90 (Control), 5.5±1.16 (Methadone-Maintained), and 7.00±1.89 (Opiate-Abstinent). Other exclusionary criteria were histories of head trauma, neurological, cardiovascular, pulmonary or systemic disease, and HIV seropositivity. The participants each received a brain MRI scan to exclude gross cerebral pathology.
Another exclusion criterion was drinking more than fifteen alcoholic beverages per week (1.5 oz liquor, 12 oz beer, or 5 oz wine). Neither moderate use of caffeine (<600 mg per day), nor occasional marijuana use (≤1 cigarette/month) were exclusionary, but we required that urine drug screens were negative for cannabinoids (See Table 2). “Experimental” use (two times or less) of stimulants, hallucinogens, or sedatives was not exclusionary.
Table 2.
Drug Use and Methadone Maintenancea
| Subject Group: | Control
(n = 13) |
Methadone-Maintained
(n = 12) |
Opiate-Abstinent
(n = 15) |
|---|---|---|---|
| Years Since Illicit Opiate Use | N/A | 5.86 ± 1.90 | 8.18 ± 2.13 |
| Years of MMT | N/A | 6.54 ± 2.43 | 7.60 ± 2.45 |
| Current Methadone Dose | N/A | 78.3 ± 5.86 | N/A |
| Years Since Methadone Detoxification | N/A | N/A | 0.91 + 0.08 |
| Typical Methadone Dose (mg/day) | N/A | 84.6 ± 5.83 | 80.8 ± 6.15
(n=11) |
| Years of Heroin Use | N/A | 15.8 ± 2.84 | 17.3 ± 2.14 |
| Peak Dollars Spent Daily on Heroin | N/A | 99.1 ± 25.0
(n=11) |
155.0 ± 35.7
(n=12) |
| Marijuana Use (Days in Last 30) | 0.07 + 0.07 | 0.15 ± 0.11 | 0.0 |
| Alcohol Use (Days in Last 7) | 2.08 ± 0.71 | 0.0 | 0.0 |
| Nicotine Use (percent smokers) | 7.7 | 66.7 | 66.7 |
| Past Cocaine Use (percent users) | 0 | 100 | 86.7 |
| Past Marijuana Use (users/total number) | 0 | 100 | 86.7 |
| Past Stimulant Use (users/total number) | 0 | 50.0 | 40.0 |
| Past Sedative Use (users/total number) | 0 | 75.0 | 73.3 |
| Hallucinogens (users/non-users) | 0 | 75.0 | 53.3 |
Data shown are means and standard errors of the means
2.2. Drug Use and Mood Assessments
To ensure drug abstinence, each subject provided samples for five urine drug screens (3 without warning) before scanning, including one on the day of the PET scan. Drug abuse history was assessed with the Substance Use Inventory (Sobell et al., 1980) (Table 2). Heroin craving was assessed with a 100-mm visual analogue scale of “intensity of desire for heroin over the past 24 hours.” Subjects completed the 20-item Cornell Dysthymia Rating Scale (CDRS) as a measure of dysphoria. Items are scored from zero (symptom occurs not at all) to four (symptom is marked in intensity or frequency), with a total possible composite score of 80 (Mason et al., 1995).
2.3. Image Acquisition
Structural MR images (1.5T GE Signa) were used to exclude gross cerebral pathology. Each subject received one SPGR 3D MRI scan of the entire brain using the following parameters: TE =13; TR = 38; field of view = 24 cm; 1.5-mm thickness; matrix 256×256; 1 excitation; flip angle = 45 degrees. One subject was excluded due to evidence of silent stroke.
For the PET studies, a thermoplastic facemask (Scrypton Systems, Annapolis, MD) was prepared individually for each subject to minimize head motion. A catheter was inserted into the antecubital vein for infusing FDG, and the participant was positioned in the scanner gantry, where a 20-min 68Ge transmission scan was performed for measured attenuation correction. The subject was then removed from the scanner, and performed an auditory continuous performance task (CPT) (version 2.26, Sunrise Systems; Pembroke, MA). This task was implemented to maintain wakefulness in a standardized fashion. The task required discrimination of a target tone (higher pitch) from a sequence (inter-stimulus interval = 2 sec) of non-target tones (lower pitch), and pressing the “x” on a computer keyboard to signify hearing a target tone. “Pink” noise (70 dB) masked ambient sounds during the FDG uptake. FDG (≤5 mCi, ≤185 MBq) was administered intravenously 3-9 min after the CPT began. The CPT was stopped 30 min after FDG administration, and the subject repositioned in the scanner.
PET scans were acquired with a Siemens ECAT EXACT HR+, with a 15.5-cm field of view. The average transverse resolutions (full width half maximum (FWHM)) at 1 and 10 cm from the center of the field of view, measured in 3D mode and determined using a fluorine-18 line source and a ramp filter (0.5 cutoff frequency), were 4.66 and 5.45 mm, respectively. The emission scan was acquired in 3D mode as six 5-min dynamic frames.
Each frame was reconstructed using the vector processor of the scanner in 3D mode by filtered back-projection (128 matrix size and zoom factor of 2.75). Scatter correction, decay correction, and attenuation correction were applied by using a Hann filter with a 0.5 cutoff frequency. The reconstructed dynamic frames were summed after they were viewed in a cine viewer to verify that there were no motion artifacts. Any frame with evidence of motion was excluded from summing.
2.4. Image Analysis
PET images were analyzed using statistical parametric mapping (SPM99, Wellcome Dept. of Cognitive Neurology, University College London). The decay-corrected PET images were converted to Analyze 7.5 volume image format and then spatially normalized into the Montreal Neurological Institute coordinate system (MNI space). The spatially transformed images contained isotropic 2-mm voxels and were smoothed with an 8×8×8 Gaussian filter.
Eight brain regions were sampled in both hemispheres based on a priori hypotheses: subgenual ACC (BA 25/32); perigenual ACC (BA 24a/32/33); mid-cingulate gyrus (BA 24); medial orbitofrontal cortex (gyrus rectus and medial orbital gyrus, BA 11); inferior parietal cortex (BA 40); insula (BA39/40); amygdala, and thalamus. Sixteen volumes of interest (VOIs) were created, using MEDx 3.42 (Medical Numerics, Sterling, VA). Each VOI was traced on a T1-weighted MRI image normalized to the same stereotaxic space as the PET images and with the same voxel dimensions. The 3-D contours of particular structures were derived from the 2-D graphics by outlining the borders and saving as a VOI. All voxels outside the VOI were set to the intensity value of 0. Thus, the search volume for group comparisons was the actual volume of the structure of interest.
Because age, CPT performance, and education varied significantly across groups, relationships between these variables and relative activity as an index of rCMRglc were first tested using covariate analysis across all subjects. Since age effects on rCMRglc in the superior frontal gyrus, ACC, and insula as well as CPT performance effects on rCMRglc in the ACC, insula and thalamus were significant, all analyses of rCMRglc were performed with age and CPT performance as nuisance covariates. Education did not yield significant correlative effects in the VOIs studied.
Group comparisons used the general linear model as implemented in SPM99. The smoothed, normalized images were proportionally scaled to the global brain mean to yield values representing relative rates of rCMRglc. Within each VOI, the voxel-wise threshold for inclusion in clusters was set at P<0.01. Furthermore, because of the contribution to the probability of false positive findings due to sampling 16 VOIs, we only considered as significant for this study those data from clusters of contiguous voxels that maintained statistical significance at P<0.01, corrected for each search volume.
We used covariate analysis to examine the relationship between relative rCMRglc and CDRS scores, as well as between relative rCMRglc and variables of heroin use history and years of MMT to identify possible cumulative opiate exposure effects. Statistical significance of the effect of each covariate was assessed within the 16 pre-selected VOIs. Again, a VOI was considered to show a significant covariate effect if it contained a cluster with P<0.01, corrected for search volume.
3. Results
3.1. Subjects
The two opiate-dependent groups were evenly matched on age, gender, race, education, and mother’s education (Table 1). They were also matched on drug abuse histories, past heroin abuse, and severity of heroin habit, indexed by money spent on heroin. The two groups did not differ on duration of MMT, methadone dose, time since using illicit drugs, past use of cocaine, stimulants, and hallucinogens, and use of marijuana, tobacco and alcohol. Both subject groups were significantly older and had fewer years of formal education than the control subjects.
3.2. Negative Mood
The differences between groups on the CDRS scores were not significant, and scores in all groups were low, consistent with euthymia, with means and standard errors of means as follows: control group 6.85 ± 1.89, MMT group 5.45 ± 0.99, and opiate-abstinent group 6.07 ± 1.42. The self-reports of heroin craving in the two opiate-dependent subject groups were all very low, with most of the participants (19/27 subjects) reporting no craving at all; therefore, data on heroin craving were not considered further.
3.3. CPT scores
Methadone-maintained subjects had worse performance than opiate-abstinent subjects whose performance, in turn, was worse than that of control subjects. Error rates in the three groups were as follows: 10.26% (Methadone-Maintained), 4.84% (Opiate-Abstinent), 0.96% (Control) (F (df2,38)=14.92; p <0.001). By Scheffe’s Pairwise Comparisons, the Methadone-Maintained (p<0.001), but not Opiate-Abstinent (p=0.078), group differed significantly from the Control group.
3.4. Relative Glucose Metabolism
Contrasted with control subjects, opiate-abstinent subjects exhibited clusters with lower relative rCMRglc in bilateral perigenual ACC, right superior frontal cortex, left insula, and left mid-cingulate cortex. No VOI showed greater relative activity in opiate-abstinent subjects compared to control subjects (Table 3). Also compared with the control subjects, the methadone-maintained participants had clusters with lower relative activity in the left thalamus and insula. Also, compared with opiate-abstinent subjects, the methadone-maintained subjects had a cluster with higher activity in the right superior frontal cortex.
Table 3.
Group Differences in Relative Activity of Pre-selected Regions Corrected for Age and CPT Performance
| Cluster-level Analysis | Peak Voxel | |||||
|---|---|---|---|---|---|---|
| Corrected P
Value |
Cluster
Size (voxels) |
Search
Volume (voxels) |
Corrected P
Value |
Z-Score | Coordinates
(x, y, z) |
|
| OA<Control | ||||||
| L ACC, perigenual | <.001 | 144 | 1091 | .016 | 3.77 | -4, 28, 18 |
| R ACC, perigenual | .004 | 79 | 1086 | .046 | 3.41 | 2, 32, 16 |
| L Mid-cingulate | .008 | 65 | 733 | .047 | 3.25 | -6, 18, 26 |
| L Insula | .007 | 80 | 1535 | .223 | 2.88 | -34, 4, 4 |
| R Superior frontal Cortex | <.001 | 385 | 6413 | .037 | 4.03 | 12, 48, 40 |
|
| ||||||
| MM<Control | ||||||
| L Insula | .004 | 89 | 1535 | .013 | 3.95 | -38, -10, 4 |
| L Thalamus | .001 | 203 | 1392 | .013 | 3.88 | -6, -20, 10 |
|
| ||||||
| MM > OA | ||||||
| R Superior frontal Cortex | <.001 | 487 | 6413 | .03 | 4.08 | 22, 46, 36 |
Note: Relative regional radioactivity (decay-corrected), as an index of regional cerebral glucose metabolism (rCMRglc), was measured in opiate-abstinent (OA) (n=15) and methadone-maintained (MM) (n=12) former heroin users and control subjects (n = 13) while they performed an auditory continuous performance task (CPT). Planned comparisons of relative activity (scaled to global mean values) were made in 8 volumes of interest (VOIs) of each hemisphere as indicated in Methods, with the a priori hypothesis of group differences. Statistical parametric maps were generated using a height threshold of P<0.01 uncorrected (voxel level). The spatial extents of activated clusters of contiguous voxels were corrected for the search volume of the relevant VOI. Corrected significant P values for cluster size (with cluster size and VOI size) and peak voxel height (with location and Z-score) are presented above. Locations for the peak voxels are given in MNI-space, with the origin set at the anterior commissure.
3.5. Covariate Analyses
In control subjects, CDRS scores covaried positively with relative rCMRglc in the right insula and negatively with activity in the right perigenual ACC (Table 4). In contrast, CDRS scores in opiate-abstinent subjects covaried positively and robustly with relative rCMRglc in a cluster in the left perigenual ACC and another in the left mid-cingulate gyrus. Lastly, in subjects receiving MMT, there were no significant correlations between rCMRglc and CDRS scores.
Table 4.
Age- and CPT-corrected Correlations of CDRS Scores Relative to Activity in Control, Opiate-Abstinent, and Methadone-Maintained Subjects
| Cluster-level Analysis | Peak Voxel | ||||||
|---|---|---|---|---|---|---|---|
| Corrected P
Value |
Cluster
Size (voxels) |
Search
Volume (voxels) |
Corrected P
Value |
Z-Score | Coordinates
(x, y, z) |
||
| Control Subjects | |||||||
| R Insula (+) | .009 | 52 | 1456 | .196 | 3.31 | 44, -2, 4 | |
| R ACC, perigenual (-) | <.001 | 116 | 1086 | .034 | 3.97 | 8, 44, 12 | |
|
| |||||||
| Opiate-Abstinent Subjects | |||||||
| L ACC, perigenual (+) | .001 | 126 | 1091 | .097 | 3.31 | -2, 60, 8 | |
| L Mid Cingulate (+) | <.001 | 150 | 733 | .086 | 3.19 | -4, 14, 32 | |
|
| |||||||
| Methadone-Maintained Subjects | |||||||
| None | N/A | N/A | N/A | N/A | N/A | N/A | |
Note: Possible relationships between depressive symptomatology and regional radioactivity (decay-corrected), as an index of relative regional cerebral glucose metabolism (rCMRglc), in opiate-abstinent, methadone-maintained , and control subjects were assessed by testing whether score on the Cornell Dysthymia Rating Scale (CDRS) was a significant covariate of activity in the 8 brain bilateral volumes of interest (VOIs), selected a priori on the basis of literature accounts of their involvement in dysphoric emotion (see Methods). These VOIs were tested with small volume correction with age and continuous performance task (CPT) score treated as nuisance covariates. Regions with clusters that exceeded the extent threshold of P < .01 corrected for search volume are listed.
No correlations were found between relative activity and measures of opiate abuse severity in either group of former heroin abusers. In methadone-maintained subjects, however, relative rCMRglc in a large area of the inferior parietal cortex (140 voxels, P=0.001, peak voxel coordinates 50, -60, 42), correlated positively with years of MMT.
4. Discussion
In this study, opiate-abstinent subjects, when compared to methadone-maintained subjects, exhibited a more widespread constellation of cerebral functional abnormalities in regions previously shown to be important in depressive states, and some of these regions correlated with dysphoric symptoms.
4.1. Dysphoric Mood
CDRS scores yielded no group differences and were low, consistent with the fact that while subjects did report some dyphoric symptoms, they were free of dysthymia or another depressive disorder.
4.2. Relative Glucose Metabolism
Opiate-abstinent subjects exhibited significantly lower relative metabolism than control subjects in a number of regions studied (see Table 3): the bilateral perigenual ACC, left mid-cingulate, left insula, and right superior frontal cortex. Hypoactivity in all of these regions except for the insula has been noted in research participants with major depression or dysphoric states and has been related to problems in regulating affect (Drevets 2000; Drevets 2000b; Drevets and Raichle 1998; Mayberg 2002). Subjects receiving MMT, on the other hand, only demonstrated lower activity in the left insula and left thalamus when compared to control subjects. The greater prominence of abnormalities in the opiate-abstinent subjects merits further research into the hypothesis that opiate substitution therapy ameliorates the mood dysregulation associated with heroin dependence.
Some of the group differences, however, did not appear to be related to mood. Relative activity in the left insula was lower for both opiate-dependent subject groups when compared to control subjects. In the right superior frontal cortex, both MMT subjects and control subjects had greater metabolic activity than opiate-abstinent subjects. In neither of these regions was there a significant relationship between rCMRglc and CDRS score. Because activity in these areas was not correlated with any of the variables studied, interpretation of these findings is necessarily limited, and additional research into the meaning of these findings is merited.
Thalamic activity in subjects receiving MMT was significantly lower than in the control group and was not correlated with CDRS score. The thalamus is a mu-opioid receptor-rich area that consistently demonstrates reduced activity during administration of opiates (London et al., 1990; Adler et al., 1997). It is therefore most likely that this result is due to direct, local opioid receptor effects of MMT.
We did not observe abnormalities of the infragenual ACG (BA25) in either of the opioid-dependent subject groups. The literature consistently indicates reversible and elevated activity in this region in depressed subjects, (Drevets 2000; Liotti et al., 2002). This difference, and lack of findings in the orbitofrontal cortex and the amygdala, may be attributed to the absence of acute depression in our participants.
4.3. Covariate Analyses
When considered in light of correlation analyses, the lower activity measured in opiate-abstinent subjects in the left perigenual ACC and left mid-cingulate may assume greater meaning. The observed significant covariation of metabolic activity with the CDRS score suggests that in opiate-abstinent individuals, these regions might play a role in the processing of dysphoric mood symptoms, even in non-depressed states. If this speculation is true, the positive direction of this correlation, which contrasts with control subjects, in concert with the metabolic abnormalities cited above, suggest that opiate-abstinent, former heroin users at baseline mood states may process negative affects differently than healthy individuals. Of note, the positive activity-CDRS correlation in this instance is counterintuitive because those opiate-abstinent subjects with greater CDRS scores exhibited greater relative activity, and therefore less difference from control subjects. However, this finding resembles observations on abstinent methamphetamine-dependent subjects, who similarly exhibited both hypoactivity of the perigenual ACC and a positive correlation between relative glucose metabolism in this area and Beck Depression Inventory score (London et al., 2004). Coupled with research demonstrating perigenual ACC hypoactivity in abstinent cannabis dependent subjects (Eldreth et al., 2004) and postmortem data documenting altered serotonin receptor density in the perigenual ACC in alcoholics (Mantere et al., 2002), these data suggest a common defect in affective processing associated with substance dependence, conferring a vulnerability to dysphoric states during abstinence from drugs of abuse.
4.4. Limitations
This study has several limitations, including the fact that the opiate-dependent subject groups differed from the control group on several variables unrelated to opiate exposure. Control subjects were younger, with higher levels of educational attainment and less past drug and tobacco use histories. Because age (though not education) did correlate with metabolic activity in some of the regions studied, we used age as a nuisance covariate in all analyses. Furthermore, because both opiate-abstinent and methadone-maintained subjects did not have significant differences in their past drug and tobacco histories, findings from comparisons of these two groups are unlikely related to these historical factors. In addition, though previous work by Daglish et al. (2001) demonstrated left anterior cingulate activation with autobiographical script-induced craving, it is unlikely that craving confounded our results, as not only were our results dissimilar in this region, but also, most subjects denied heroin craving entirely, and only one subject was removed from the study because of a positive urine drug test.
Furthermore, CDRS scores across all subjects were low, which, although beneficial in confirming the absence of dysthymia or major depression, contributed to a narrow distribution of scores. Thus, the meaning of increments between subjects and of the subsequent correlation analysis must be interpreted carefully, and this may also have constrained the ability to detect correlations that might have been found otherwise in a larger sample. However, the fact that we were able to demonstrate a significant correlation in opiate-abstinent opiate-dependent subjects despite the restricted range of scores strengthens this particular result.
As mentioned, our data may be confounded by selection bias, as subjects were not randomized to their groups. Rather, during the course of treatment, subjects decided whether to enroll in the abstinent treatment program or to continue MMT. In addition, our subjects were all treatment-compliant, motivated, and in highly-structured programs; accordingly, our results may not be generalizable to all opiate-dependent clients.
There is a possibility that the CPT may have affected our study groups differently. We accounted for CPT performance in all analyses to minimize this risk, but must acknowledge that unmeasured cognitive processes may have affected brain activity. Finally, the group differences we observed could be related solely to unmeasured variables, such as nutritional status or psychosocial stressors.
4.5. Summary
In conclusion, both methadone-maintained and opiate-abstinent heroin-dependent subjects in sustained remission exhibited abnormalities in regional brain activity. These abnormalities were more pronounced in the opiate-abstinent subjects, in whom lower activity in the perigenual anterior cingulate and mid-cingulate cortices, in particular, was related to dysphoria. When compared to healthy individuals, clients receiving MMT only demonstrated lower activity in the left insula and thalamus, which was not clearly related to mood. The findings, therefore support the view that methadone may be useful for mitigating aberrant mood processing even in non-depressed individuals with remitted opiate dependence. Larger, prospective studies are warranted to assess whether these differences are indeed effects of treatment modality and thereby augment our current understanding of the neurobiological sequelae of choosing between continued methadone treatment and opiate abstinence in this population.
Figure 1.
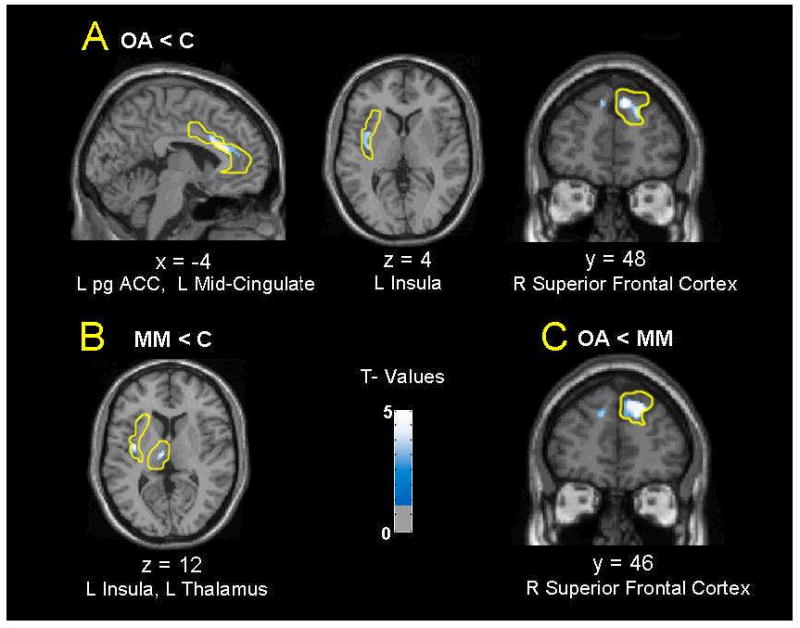
Locations of differences between opiate abstinent (OA), methadone maintained (MM), and control (C) groups in relative activity reflecting regional cerebral glucose metabolism (rCMRglc), based on analyses of 8 bilateral preselected volumes of interest (VOIs) in each hemisphere as indicated in Methods, with the a priori hypothesis of group differences. Age and continuous performance task (CPT) performance were used as nuisance variables, and statistical parametric maps were generated for each VOI using a height threshold of P<0.01 uncorrected. Coordinates of sections shown are in MNI-space, with the origin set at the anterior commissure. (pgACC = perigenual anterior cingulate cortex)
Figure 2.
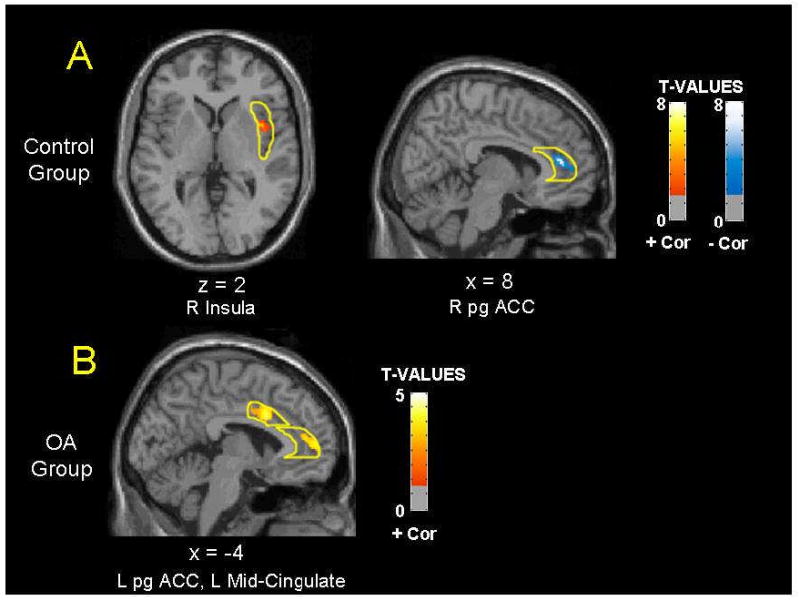
Locations of covariation between Cornell Dysthymia Rating Scale scores and relative regional cerebral glucose metabolism (rCMRglc) in opiate abstinent (OA) and control groups (no covariation existed in the methadone-maintained group) based on small volumes correction analyses of 8 pre-selected volumes of interest (VOIs) in each hemisphere, as indicated in Methods. Age and continuous performance task (CPT) performance were used as nuisance variables, and statistical parametric maps were generated for each VOI using a height threshold of P<0.01 uncorrected. Positive correlations are shown in red, while negative correlations are shown in blue. Coordinates of sections shown are in MNI-space, with the origin set at the anterior commissure. (pgACC = perigenual anterior cingulate cortex)
Acknowledgments
The authors thank Drs. Don Des Jarlais and Christian Miner for conceptual help in the early stages of this project. We also thank Denise Dunovant MA, James Quick CASAC and the staff of Su Casa residential treatment facility (Lower Eastside Service Center, Inc.) for invaluable assistance with subject recruitment. We are grateful to Ms. Carrie Weaver for her superb organizational work in the course of this project. We thank Dr. Andrew Horti, Morgan Stratton and Andrew Hall for preparing the radiotracer and Janet Kivett and the NIDA nursing staff for their assistance during PET scanning.
Preliminary reports have been presented at annual meetings of the American College of Neuropsychopharmacology, San Juan, Puerto Rico, December 11-15, (2000), and the American Psychiatric Association, San Francisco, CA, May 18-23 (2002)
This study was supported in part by RO1 DA 12273 (to Dr. Galynker), the NIDA Intramural Research Program, and the Counterdrug Technology Center, Office of National Drug Control Policy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler LJ, Gyulai FE, Diehl DJ, Mintun MA, Winter PM, Firestone LL. Regional brain activity changes associated with fentanyl analgesia elucidated by positron emission tomography. Anesth Analg. 1997;84:120–6. doi: 10.1097/00000539-199701000-00023. [DOI] [PubMed] [Google Scholar]
- Ball JC, Ross A. The Effectiveness of Methadone Maintenance Treatment. New York: Springer-Verlag; 1991. [Google Scholar]
- Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Britten S, Brewer C, Lingford-Hughes A, Myles JS, Grasby P, Nutt DJ. Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. Am J Psychiatry. 2001;158:1680–1686. doi: 10.1176/appi.ajp.158.10.1680. [DOI] [PubMed] [Google Scholar]
- Danos P, Kasper S, Grunwald F, Klemm E, Krappel C, Broich K, Hoflich G, Overbeck B, Biersack HJ, Moller HJ. Pathological regional cerebral blood flow in opiate-dependent patients during withdrawal: a HMPAO-SPECT study. Neuropsychobiology. 1998;37:194–199. doi: 10.1159/000026502. [DOI] [PubMed] [Google Scholar]
- Darke S, Ross J. Suicide among heroin users: rates, risk factors and methods. Addiction. 2002;97:1383–94. doi: 10.1046/j.1360-0443.2002.00214.x. [DOI] [PubMed] [Google Scholar]
- Dean AJ, Bell J, Christie MJ, Mattic RP. Depressive symptoms during buprenorphine vs. methadone maintenance: findings from a randomised, controlled trial in opioid dependence. European Psychiatry. 2004;19:510–513. doi: 10.1016/j.eurpsy.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Miller HL, Salomon RM, Licinio J, Krystal JH, Moreno FA, Heninger GR, Charney DS. Tryptophan-depletion challenge in depressed patients treated with desipramine or fluoxetine: implications for the role of serotonin in the mechanism of antidepressant action. Biol Psychiatry. 1999;46:212–220. doi: 10.1016/s0006-3223(99)00014-1. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000b;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition and Emotion. 1998;12:353–385. [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–20. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- First M, Gibbon M, Spitzer R, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders (Research version) New York, NY: New York State Psychiatric Institute, Biometrics Research Department; 1996. [Google Scholar]
- Flynn PM, Joe GW, Broome KM, Simpson DD, Brown BS. Recovery from opioid addiction in DATOS. J Subst Abuse Treat. 2003;25:177–186. doi: 10.1016/s0740-5472(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Galynker II, Watras-Ganz S, Miner C, Rosenthal RN, Des J, Richman BL, London ED. Cerebral metabolism in opiate-dependent subjects: effects of methadone maintenance. Mt Sinai J Med. 2000;67:381–387. [PubMed] [Google Scholar]
- Gossop M, Stewart D, Browne N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction. 2002;97:1259–1267. doi: 10.1046/j.1360-0443.2002.00227.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W. Frontal gamma-band activity in magnetoencephalogram during auditory oddball processing. Neuroreport. 2004;15:2185–8. doi: 10.1097/00001756-200410050-00008. [DOI] [PubMed] [Google Scholar]
- Kaufman MJ, Pollack MH, Villafuerte RA, Kukes TJ, Rose SL, Mendelson JH, Cohen BM, Renshaw PF. Cerebral phosphorus metabolite abnormalities in opiate-dependent polydrug abusers in methadone maintenance. Psychiatry Res. 1999;90:143–152. doi: 10.1016/s0925-4927(99)00017-7. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Woods SW, Kosten TR, Rosen MI, Seibyl JP, van Dyck CC, Price LH, Zubal IG, Hoffer PB, Charney DS. Opiate dependence and withdrawal: preliminary assessment using single photon emission computerized tomography (SPECT) Am J Drug Alcohol Abuse. 1995;21:47–63. doi: 10.3109/00952999509095229. [DOI] [PubMed] [Google Scholar]
- Latowsky M. Improving detoxification outcomes from methadone maintenance treatment: the interrelationship of affective states and protracted withdrawal. J Psychoactive Drugs. 1996;28:251–257. doi: 10.1080/02791072.1996.10472486. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15:1028–38. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatry. 2002;159:1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, Thompson PM, Brody AL, Geaga JA, Hong MS, Hayashi KM, Rawson RA, Ling W. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry. 2005;58:770–8. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- London ED, Broussolle EP, Links JM, Wong DF, Cascella NG, Dannals RF, Sano M, Herning R, Snyder FR, Rippetoe LR. Morphine-induced metabolic changes in human brain. Studies with positron emission tomography and [fluorine 18]fluorodeoxyglucose. Arch Gen Psychiatry. 1990;47:73–81. doi: 10.1001/archpsyc.1990.01810130075010. [DOI] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- Mantere T, Tupala E, Hall H, Sarkioja T, Rasanen P, Bergstrom K, Callaway J, Tiihonen J. Serotonin transporter distribution and density in the cerebral cortex of alcoholic and nonalcoholic comparison subjects: a whole-hemisphere autoradiography study. Am J Psychiatry. 2002;159:599–606. doi: 10.1176/appi.ajp.159.4.599. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Kocsis JH, Leon AC, Thompson S, Frances AC, Morgan RO, Parides MK. In: Assessment of symptoms and change in dysthymic disorder in Diagnosis and treatment of chronic depression. Kocsis JH, Klein DN, editors. New York, NY: Guilford Press; 1995. [Google Scholar]
- Mason BJ, Kocsis JH, Melia D, Khuri ET, Sweeney J, Wells A, Borg L, Millman RB, Kreek MJ. Psychiatric comorbidity in methadone maintained patients. J Addict Dis. 1998;17:75–89. doi: 10.1300/J069v17n03_07. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Depression, II: Localization of Pathophysiology. Am J Psychiatry. 2002;159:1979. doi: 10.1176/appi.ajp.159.12.1979. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McKeever WF, Seitz KS, Krutsch AJ, Van Eys PL. On language laterality in normal dextrals and sinistrals: results from the bilateral object naming latency task. Neuropsychologia. 1995;33:1627–1635. doi: 10.1016/0028-3932(95)00042-9. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Kell MJ. Prevalence and improvement in psychopathology in opioid dependent patients participating in methadone maintenance. J Addict Dis. 1995;14:67–82. doi: 10.1300/J069v14n03_05. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Sullivan MA, Levin FR. Treatment of depression in patients with opiate dependence. Biol Psychiatry. 2004;56:793–802. doi: 10.1016/j.biopsych.2004.06.037. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Fischer G, Podreka I, Schindler S, Brucke T, Jagsch R, Thurnher M, Kasper S. Opioid addiction changes cerebral blood flow symmetry. Neuropsychobiology. 2002;45:67–73. doi: 10.1159/000048679. [DOI] [PubMed] [Google Scholar]
- Rao SR, Broome KM, Simpson DD. Depression and hostility as predictors of long-term outcomes among opiate users. Addiction. 2004;99:579–589. doi: 10.1111/j.1360-0443.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- Rose JS, Branchey M, Buydens-Branch, Stapleton JM, Chasten K, Werrell A, Maayan ML. Cerebral perfusion in early and late opiate withdrawal: a technetium-99m-HMPAO SPECT study. Psychiatry Res. 1996;67:39–47. doi: 10.1016/0925-4927(96)02663-7. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Jones C, Novelly RA, Kleber H. Neuropsychological functioning in opiate addicts. J Nerv Ment Dis. 1982;170:209–216. doi: 10.1097/00005053-198204000-00005. [DOI] [PubMed] [Google Scholar]
- Satel SL, Kosten TR, Schuckit MA, Fischman MW. Should protracted withdrawal from drugs be included in DSM-IV? Am J Psychiatry. 1993;150:695–704. doi: 10.1176/ajp.150.5.695. [DOI] [PubMed] [Google Scholar]
- Sarikaya A, Karasin E, Cermik TF, Abay E, Berkarda S. Evaluation of dysthymic disorder with technetium-99 m hexamethylpropylene amine oxime brain single-photon emission tomography. Eur J Nucl Med. 1999;26:260–264. doi: 10.1007/s002590050386. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Maisto SA, Sobell LC, Cooper AM, Cooper T, Sanders B. Developing a prototype for evaluating alcohol treatment outcome studies. In: Sobell LC, Sobell MD, Ward E, editors. Evaluating Alcohol and Drug Abuse Treatment Effectiveness: Recent Advances. New York: Pergamon Press; 1980. pp. 129–150. [Google Scholar]
- Woods SW, O’Malley SS, Martini BL, Mcdougle CJ, Price LH, Krystal JH, Hoffer PB, Kosten TR. SPECT regional cerebral blood flow and neuropsychological testing in non-demented HIV-positive drug abusers: Preliminary results. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:649–662. doi: 10.1016/0278-5846(91)90055-6. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Dannals RF, Frost JJ. Gender and age influences on human brain muopioid receptor binding measured by PET. Am J Psychiatry. 1999;156:842–848. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]


