Abstract
Paclitaxel is a commonly used cancer chemotherapy drug that frequently causes painful peripheral neuropathies. The mechanisms underlying this dose-limiting side effect are poorly understood. Growing evidence supports that proinflammatory cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor (TNF), released by activated spinal glial cells and within the dorsal root ganglia (DRG) are critical in enhancing pain in various animal models of neuropathic pain. Whether these cytokines are involved in paclitaxel-induced neuropathy is unknown. Here, using a rat neuropathic pain model induced by repeated systemic paclitaxel injections, we examined whether paclitaxel upregulates proinflammatory cytokine gene expression, and whether these changes and paclitaxel-induced mechanical allodynia can be attenuated by intrathecal IL-1 receptor antagonist (IL-1ra) or intrathecal delivery of plasmid DNA encoding the anti-inflammatory cytokine, interleukin-10 (IL-10). The data show that paclitaxel treatment induces mRNA expression of IL-1, TNF, and immune cell markers in lumbar DRG. Intrathecal IL-1ra reversed paclitaxel-induced allodynia and intrathecal IL-10 gene therapy both prevented, and progressively reversed, this allodynic state. Moreover, IL-10 gene therapy resulted in increased IL-10 mRNA levels in lumbar DRG and meninges, measured 2 weeks after initiation of therapy, whereas paclitaxel-induced expression of IL-1, TNF, and CD11b mRNA in lumbar DRG was markedly decreased. Taken together, these data support that paclitaxel-induced neuropathic pain is mediated by proinflammatory cytokines, possibly released by activated immune cells in the DRG. We propose that targeting the production of proinflammatory cytokines by intrathecal IL-10 gene therapy may be a promising therapeutic strategy for the relief of paclitaxel-induced neuropathic pain.
Keywords: glia, neuropathic pain, macrophages, interleukin-1, tumor necrosis factor, meninges
Introduction
The chemotherapeutic drug paclitaxel (Taxol®) frequently induces painful peripheral neuropathies (Dougherty et al., 2004). The mechanisms underlying this dose-limiting side effect are unclear and few drugs prevent or control it. Available drug therapies primarily target neurons. However, in the past decade, data strongly support that proinflammatory cytokines, in particular interleukin-1 (IL-1) and tumor necrosis factor (TNF), produced by activated glia (microglia and astrocytes), are critical in the creation and maintenance of neuropathic pain in animal models (Marchand et al., 2005; Moalem and Tracey, 2006; Watkins and Maier, 2003).
Targeting glial proinflammatory cytokines may therefore provide an effective approach for the control of neuropathic pain. A promising candidate therapeutic is the anti-inflammatory cytokine interleukin-10 (IL-10), a powerful suppressor of the production and activity of several proinflammatory mediators (Moore et al., 2001; Strle et al., 2001). Indeed, IL-10 has been reported to be effective in the control of acute and chronic pain. Intrathecal IL-10 protein blocks the development of IL-1-mediated pain changes induced by intrathecal dynorphin (Laughlin et al., 2000) and by peri-sciatic snake venom phospholipase A2 (Chacur et al., 2004). Intrathecal IL-10 protein also reverses sciatic nerve chronic constriction injury (CCI)-induced mechanical allodynia and thermal hyperalgesia (Milligan et al., 2005a). In addition, intrathecal administration of adenoviral or adeno-associated viral vectors containing cDNA encoding IL-10 prevented and/or reversed mechanical allodynia induced by intrathecal. HIV-1 gp120, sciatic nerve inflammation (sciatic inflammatory neuropathy, SIN), and CCI (Milligan et al., 2005a; Milligan et al., 2005b). More recently, we have been pursuing intrathecal delivery of ‘naked’ plasmid DNA encoding rat IL-10, which results in a far more prolonged reversal of CCI-induced allodynia (Milligan et al., 2006).
Recently, a rat model has been described wherein repeated intraperitoneal injections of low doses of paclitaxel induce stable mechanical allodynia, but no significant systemic toxicity or motor impairment (Polomano et al., 2001). The behavioral responses last for several weeks to months (Flatters and Bennett, 2004; Polomano et al., 2001), thus modeling painful neuropathies in patients that can persist for months after termination of chemotherapy. Pain enhancement in this model is relatively resistant to opioid treatment and does not appear to involve activation of NMDA receptors (Flatters and Bennett, 2004). In contrast, ethosuximide, an anti-epileptic and calcium channel blocker, was effective in reversing mechanical allodynia/hyperalgesia in this model (Flatters and Bennett, 2004). Whether proinflammatory cytokines contribute to paclitaxel-induced neuropathic pain is as yet unknown.
Thus, the purpose of the present study is to assess whether paclitaxel-induced neuropathic pain in rats is mediated by pro-inflammatory cytokines, and is associated with spinal cord glial activation. We therefore examined whether paclitaxel-induced low-threshold mechanical allodynia is associated with increases in the expression of spinal glial activation markers and whether it can be reversed by intrathecal IL-1 receptor antagonist (IL-1ra) and/or intrathecal IL-10 gene therapy. In addition, the present study explores whether these putative pain modulatory effects are associated with changes in proinflammatory cytokine expression in spinal cord, meninges, and/or dorsal root ganglia.
Materials and Methods
Subjects
Pathogen-free adult male Sprague-Dawley rats (325-375 g; Harlan Labs, Madison, WI) were used in all experiments. Rats were housed in temperature (23 ± 3 °C) and light (12 h: 12 h light:dark cycle; lights on at 7 AM) controlled rooms with standard rodent chow and water available ad libitum. Behavioral testing and injections were performed during the light cycle. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Colorado at Boulder.
Drugs and plasmid DNA
Paclitaxel (Taxol®; Bristol-Myers Squibb, Princeton, NJ; 6 mg/ml in Cremophor EL/ethanol 1:1) was diluted in 0.9% sterile saline to a concentration of 1 or 2 mg/ml prior to injection. The vehicle was a 1:1 mixture of Cremophor EL/ethanol diluted in saline as above. Endotoxin-free solutions of recombinant human interleukin-1 receptor antagonist (IL-1ra; 100 μg/μl) and its vehicle were kindly provided by Amgen (Thousand Oaks, CA) and stored at 4°C. Plasmid DNA encoding for rat interleukin-10 (pDNA-IL-10) was identical to the expression cassette used in our previous studies with adeno-associated virus, encoding the rat IL-10 gene with a point mutation (F129S) under the control of a hybrid cytomegalovirus (CMV) enhancer/chicken β-actin promoter (Milligan et al., 2005b). Here it was used in the absence of the viral vector; that is, as naked DNA. The control plasmid (pDNA-Control) was constructed from the pDNA-IL-10 plasmid by enzymatic excision of the IL-10 gene and insertion of a poly-A sequence thereby keeping all other elements of the backbone identical. Plasmids were amplified in SURE2 cells (Stratagene, La Jolla, CA), and purified using an Endotoxin-Free Qiagen Giga purification kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions. Purified plasmid DNA was suspended in sterile Dulbecco’s phosphate-buffered saline (PBS; 0.1 micron pore-filtered, pH 7.2; Gibco, Grand Island, NY) with 3% sucrose (Sigma, St. Louis, MO) and stored at -20 °C. Concentration and purity of plasmid DNA solutions were determined by measuring the absorbance at 260 nm and 280 nm in a spectrophotometer. Final concentration of plasmid solutions ranged from 5-6 μg/μl.
Drug administration and experimental procedures
Paclitaxel (1 or 2 mg/kg per injection) or vehicle was administered intraperitoneally (i.p.) on four alternate days (Days 0, 2, 4, and 6; cumulative dose 4 or 8 mg/kg), as described previously (Polomano et al., 2001).
Acute intrathecal (i.t.) injections were performed under brief isoflurane anesthesia (3% in oxygen) using an 18-gauge sterile needle, temporarily inserted between lumbar vertebrae L5 and L6, as a guide for a PE10 injection catheter, to allow injection of compounds at the level of the lumbosacral enlargement, as described previously (Ledeboer et al., 2005). Drugs were injected over ∼10-20 sec. IL-1ra was administered in a dose of 150 μg in 1.5 μl followed by an 8 μl saline flush. Plasmid DNA was given in a final injection volume of 16-20 μl, either undiluted (for 100 μg dose) or 1:4 diluted in PBS/3% sucrose (for 25 μg dose). No abnormal motor behaviors were observed following any injection.
The details for each experiment are listed in Table 1. In all experiments, the first administration of paclitaxel or its vehicle was given on Day 0, and response thresholds of the hind paws to touch/pressure stimuli (von Frey test) were assessed as indicated in the figures for each experiment. In Experiment 2, 2 rats of each group were perfused on either Day 35 or 42 and tissues were collected for immunohistochemistry analysis. In Experiment 7, animals were sacrificed on Day 36 and tissues were collected for RT-PCR analysis.
Table 1.
Experimental design
| Exp. # | n/group | Paclitaxel dose (cumulative) | Intrathecal treatment | Day(s) of injection (Dose in μg) |
|---|---|---|---|---|
| 1 | 3-4 | 4 or 8 mg/kg | - | - |
| 2 | 4 | 8 mg/kg | - | - |
| 3 | 6-7 | 4 mg/kg | IL-1ra or vehicle | 18 (150) |
| 4 | 5 | 4 mg/kg | pDNA-IL-10 or PBS | 35 (100), 38 (25) |
| 5 | 5-6 | 4 mg/kg | pDNA-IL-10, pDNA-Control, or PBS | 12 (100), 15 (25) |
| 6 | 5 | 8 mg/kg | pDNA-IL-10, pDNA-Control, or PBS | 35 (100), 38 (25), 78(25) |
| 7 | 4-8 | 4 mg/kg | pDNA-IL-10, pDNA-Control, or PBS | 18 (100), 21 (25) |
von Frey test for mechanical allodynia
The von Frey test (Chaplan et al., 1994) was performed within the sciatic innervation area of the hind paws as previously described (Milligan et al., 2000). Briefly, calibrated Semmes-Weinstein monofilaments (von Frey hairs; Stoelting, Wood Dale, IL) were applied randomly to left and right hind paws to elicit paw withdrawal responses. The monofilaments used ranged from 3.61 (0.407 g) to 5.18 (15.136 g) bending force. Assessments were made prior to (baseline) and at specific times after drug injections, as detailed below for each experiment. Behavioral testing was performed blind with respect to drug administration. The behavioral responses were used to calculate the 50% paw withdrawal threshold (absolute threshold), by fitting a Gaussian integral psychometric function using a maximum-likelihood fitting method, allowing parametric statistical analyses (Harvey, 1986; Treutwein and Strasburger, 1999), as described in detail previously (Milligan et al., 2000). Paclitaxel treatment results in bilateral allodynia (data not shown). Because thresholds did not differ between left and right hind paws at any time point in any group, values from both paws were averaged for further analyses and data presentation.
Tissue collection
Upon completion of behavioral testing in Experiment 2 (see below), rats were anesthetized with sodium pentobarbital (Abbot Laboratories, North Chicago, IL) and transcardially perfused with saline, followed by 4% paraformaldehyde (PFA)/0.1 M phosphate buffer (PB; pH 7.4). The spinal cord was dissected, post-fixed overnight at 4°C, and segments of ∼5 mm were embedded in gelatin blocks. These blocks were then fixed overnight at 4°C in 4% PFA/PB, cryoprotected in 25% sucrose/PB, and sectioned on a cryostat at 35 μm. Upon completion of behavioral testing in Experiment 7, rats were perfused with ice-cold, isotonic saline. Lumbar spinal cord segments of ∼3-5 mm were removed, divided in left and right halves (each half containing both dorsal and ventral parts), and flash frozen in liquid nitrogen. Lumbar meninges, and left and right L4-6 dorsal root ganglia (DRG) were also removed and flash frozen in liquid nitrogen. Samples were stored at -80°C until assayed.
Immunohistochemistry
Immunoreactivity for the microglial activation markers OX-42 (labels complement type 3 receptors; CD11b) and OX-6 (labels major histocompatibility complex [MHC] class II), and the astrocyte activation marker glial fibrillary acidic protein (GFAP), was assessed. Sections were treated with 0.3% H2O2 in Tris-buffered saline (TBS) for 20 min at room temperature to suppress endogenous peroxidase activity. Sections were then incubated overnight at 4°C in monoclonal mouse anti-rat OX-42 (1:1,000; Pharmingen, San Diego, CA), mouse anti-rat OX-6 (1:1,000; Serotec, Oxford, UK), or polyclonal rabbit anti-rat GFAP antibody (1:2,000; DAKO, Carpinteria, CA) in TBS with 3% normal goat serum and 0.5% Triton-X-100. Subsequently, sections were incubated with the appropriate secondary biotinylated antibodies (1:400; Jackson ImmunoResearch, West Grove, PA) for 2 h at RT, incubated in avidin-biotin complex solution (ABC; 1:400; Vector Laboratories, Burlingame, CA) for 1 h at RT, followed by reaction with 0.5 mg/ml 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma). Finally, sections were mounted on gelatin-coated slides, dried, dehydrated, and coverslipped with Permount. Staining was evaluated by light microscopy.
RNA isolation and cDNA synthesis
Total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Samples were treated with DNase (Invitrogen) to remove contaminating DNA, and concentration and purity were determined by measuring the absorbance at 260 nm and 280 nm in a spectrophotometer.
Total RNA was reverse transcribed into cDNA using the Superscript II First-Strand Synthesis System from Invitrogen. cDNA was synthesized using 1 μg of total RNA, 50 ng of random primers, 0.5 mM dNTP mix, 10 mM dithiothreitol, 1x RT buffer and 200 U of SuperScript II reverse transcriptase in a total volume of 20 μl. The reaction was carried out at 42°C for 50 min and terminated by deactivation of the enzyme at 70°C for 15 min. Control reactions lacking either reverse transcriptase or template were included to assess carryover of genomic DNA and non-specific contamination, respectively.
Real-Time Polymerase Chain Reaction (PCR)
Amplification of cDNA was performed using the QuantiTect SYBR Green PCR Kit (Qiagen) on a MyiQ Single Color Real-Time PCR Detection System (Bio-Rad, Hercules, CA), as described previously (Ledeboer et al., 2005). Primer specifications are listed in Table 2. The threshold cycle (CT, the number of cycles to reach threshold of detection) was determined for each reaction, and the levels of the target mRNAs were quantified relatively to the level of the housekeeping gene glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) using the comparative CT (Δ CT) method (Livak and Schmittgen, 2001).
Table 2.
Oligonucleotide primers used for the amplification of rat cDNAa
| Gene | GenBank Accession no. | Sequence (5′- 3′) |
|---|---|---|
| GAPDH | M17701 | GTTTGTGATGGGTGTGAACC (forward) |
| TCTTCTGAGTGGCAGTGATG (reverse) | ||
| CD11b | NM_012711 | CTGGGAGATGTGAATGGAG (forward) |
| ACTGATGCTGGCTACTGATG (reverse) | ||
| GFAP | AF028784 | AGGGACAATCTCACACAGG (forward) |
| GACTCAACCTTCCTCTCCA (reverse) | ||
| MHC IIα | AJ554216 | AGAGACCATCTGGAGACTTG (forward) |
| CATCTGGGGTGTTGTTGGA (reverse) | ||
| IL-1β | M98820 | GAAGTCAAGACCAAAGTGG (forward) |
| TGAAGTCAACTATGTCCCG (reverse) | ||
| TNF-α | D00475 | CTTCAAGGGACAAGGCTG (forward) |
| GAGGCTGACTTTCTCCTG (reverse) | ||
| IL-10b | NM_012854 | TAAGGGTTACTTGGGTTGCC (forward) |
| TATCCAGAGGGTCTTCAGC (reverse) | ||
| pIL-10c | NM_012854 | CAGTGGAGCAGGTGAAGA (forward) |
| CCGCCAGTGTGATGGATA (reverse) |
Primers were designed using the Qiagen Oligo Analysis & Plotting Tool, and were purchased from Proligo (Boulder, CO, USA).
Detects ‘total’ IL-10, i.e. both endogenous and plasmid-derived IL-10 mRNA.
Detects only plasmid-derived IL-10 mRNA.
Statistical analysis
All statistical comparisons were computed using Statview 5.0.1 for the Macintosh. Data from the von Frey test were analyzed as the interpolated 50% threshold (absolute threshold) in log10 of stimulus intensity (monofilament stiffness in mg × 10). Baseline measures for the von Frey test were analyzed by one-way ANOVA. Time-course measures were analyzed by repeated measures ANOVA followed by Fisher’s protected least significant difference post-hoc comparisons, where appropriate. PCR data were analyzed by two-way ANOVA, followed by Fisher’s protected least significant difference post-hoc comparisons, where appropriate. If necessary, data were log-transformed prior to analysis. P values < 0.05 were considered to indicate a significant difference.
Results
Experiment 1: Time course of paclitaxel-induced mechanical allodynia
To establish the relative magnitude and duration of effects using different paclitaxel doses, mechanical allodynia was assessed using the von Frey test in rats following i.p. vehicle or paclitaxel (separate groups receiving a cumulative dose of 4 or 8 mg/kg). Both paclitaxel-treated groups developed reliable bilateral allodynia between Days 14-21 (Fig. 1). Mechanical allodynia lasted for approximately 6 weeks in the 4 mg/kg group (p<0.05 vs. vehicle for Days 24-63, 78), and about 16 weeks in the 8 mg/kg group (p<0.05 vs. vehicle for Days 24-140), consistent with previous observations (Flatters and Bennett, 2006). In contrast, vehicle-treated rats did not exhibit significant allodynia. Paclitaxel-treated rats exhibited normal posture, grooming and locomotor behavior, and gained body weight normally, comparable to vehicle-injected rats. Thus, the duration of the allodynia appears to be dose-dependent, though the magnitude of allodynia did not differ between groups that received low and high doses of paclitaxel, consistent with previous reports (Polomano et al., 2001; Smith et al., 2004).
Fig. 1.
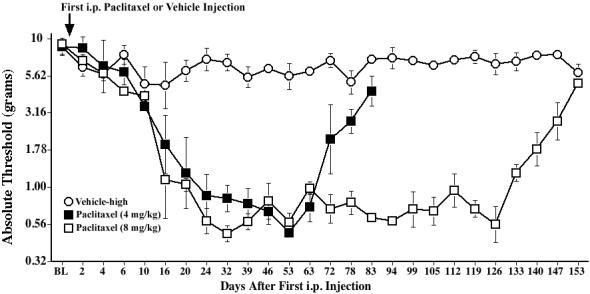
Time course of mechanical allodynia induced by repeated administration of paclitaxel. Rats received 4 i.p. injections of paclitaxel (1 or 2 mg/kg) on alternate days for a cumulative dose of 4 or 8 mg/kg, respectively, and low-threshold mechanical sensitivity was assessed by the von Frey test, before (baseline, BL), and up to Day 153. Data represent means ± SE.
Experiment 2: Effect of paclitaxel on glial cell immunoreactivity in spinal cord
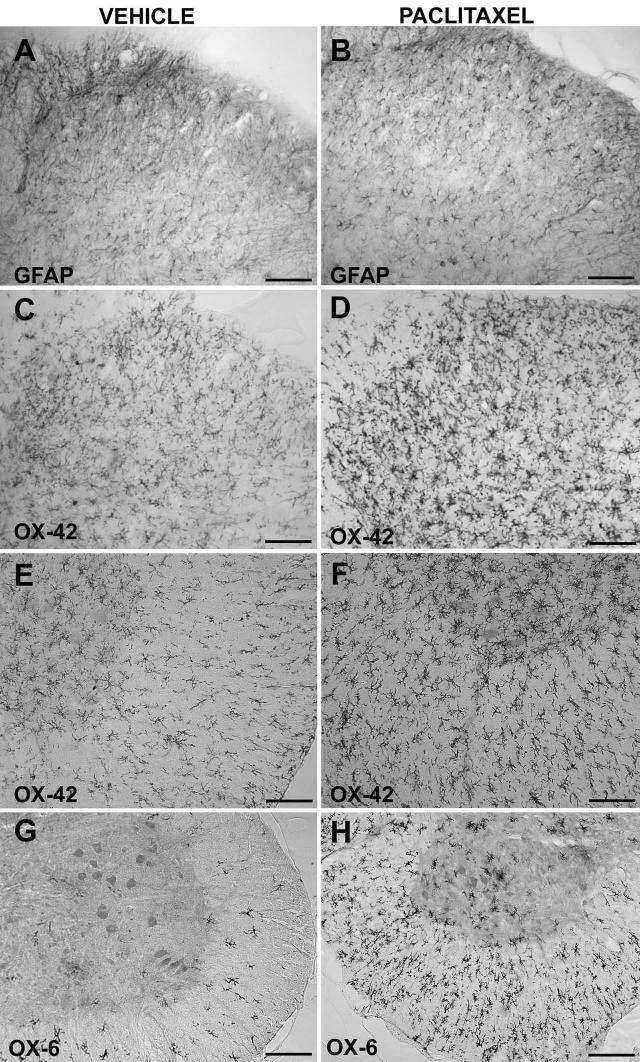
To examine whether paclitaxel leads to spinal microglial and/or astroglial cell activation, immunohistochemistry was performed on lumbar and cervical spinal cord of rats treated with vehicle or paclitaxel (cumulative dose 8 mg/kg). Behavioral assessments confirmed stable withdrawal thresholds in vehicle-injected rats, and development of bilateral mechanical allodynia in paclitaxel-injected rats (data not shown). As shown in Fig. 2A, B, the dorsal horn of lumbar (L5/L6) spinal cord of paclitaxel-treated rats showed no evident differences in GFAP immunoreactivity in vehicle- and paclitaxel-treated animals. However, paclitaxel injections resulted in increased OX-42 immunoreactivity throughout the lumbar spinal cord, both in the dorsal horn (Fig. 2C, D) and in the ventral horn and white matter (Fig. 2E, F). In addition, a higher number of OX-6-positive cells were observed throughout the white matter, and in the gray matter predominantly in the ventral horn in paclitaxel-treated rats, compared to vehicle-injected animals (Fig. 2G, H). Similar changes were observed in the cervical spinal cord (data not shown). Thus, paclitaxel appears to induce microglial, but not astroglial, activation, throughout the spinal cord 5-6 weeks after initiation of paclitaxel dosing.
Fig. 2.
Repeated administration of paclitaxel increases expression of the microglial activation markers OX-42 and OX-6, but not of the astrocyte marker GFAP, in lumbar (L5/L6) spinal cord. Rats received i.p. injections of vehicle or paclitaxel (cumulative dose 8 mg/kg) on alternate days, and were sacrificed on Day 35 or 42 to collect tissues for immunohistochemistry analysis. Representative photomicrographs are shown of GFAP (A, B) and OX-42 (C, D) immunoreactivity in the dorsal horn, and of OX-42 (E, F) and OX-6 (G, H) immunoreactivity in the ventral part of the lumbar spinal cord of rats treated with vehicle (A, C, E, G) or paclitaxel (B, D, F, H). Scale bar is 100 μm in E and F, and 200 μm in A-D, G, H.
Experiment 3: Reversal of paclitaxel-induced mechanical allodynia by i.t. IL-1ra
To examine the involvement of spinal proinflammatory cytokines in paclitaxel-induced mechanical allodynia, we first assessed the efficacy of i.t. administration of IL-1ra protein. IL-1ra has previously been used in various models of pain facilitation, including inflammatory and neuropathic pain, to examine the involvement of IL-1/IL-1 receptors (Chacur et al., 2004; Laughlin et al., 2000; Milligan et al., 2003; Watkins et al., 1997). Baseline assessments on the von Frey test revealed no differences between groups (Fig. 3). Repeated i.p. injections of paclitaxel (cumulative dose 4 mg/kg) induced reliable bilateral allodynia by Day 14 after the first injection. I.t. IL-1ra (150 μg), administered after the Day 18 test, transiently reversed mechanical allodynia in both hind paws (p<0.05 vs. vehicle for 1-3 h), with maximal reversal observed 2 h after IL-1ra injection. Allodynia fully returned by 24 h. These results implicate the spinal proinflammatory cytokine IL-1 in mediating paclitaxel-induced allodynia.
Fig. 3.
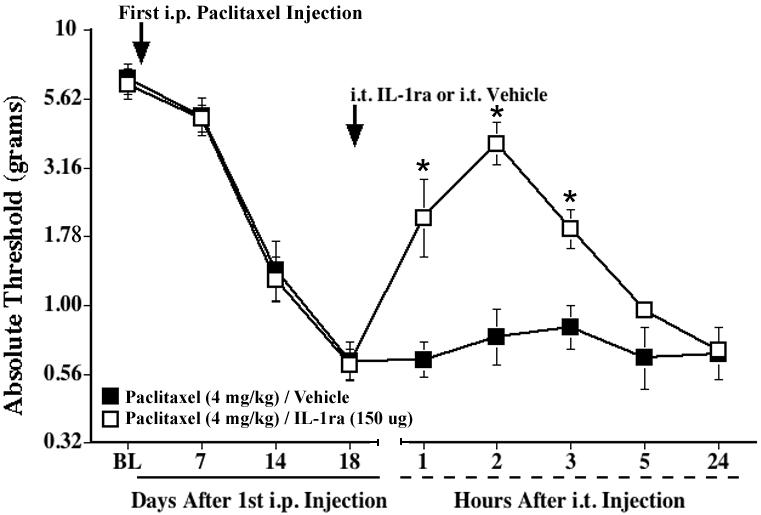
A single i.t. injection of IL-1ra transiently reverses paclitaxel-induced mechanical allodynia. Rats received i.p. injections of paclitaxel (cumulative dose 4 mg/kg) on alternate days, and IL-1ra (150 μg) or vehicle was administered intrathecally 18 days after the first paclitaxel injection (indicated by the arrow). Low-threshold mechanical sensitivity was assessed by the von Frey test, before (baseline, BL), on Day 18, and 1, 2, 3, 5, and 24 h after the i.t. injection. Data represent means ± SE. *p<0.05 vs. paclitaxel/vehicle.
Experiment 4: Reversal of paclitaxel-induced mechanical allodynia by i.t. pDNA-IL-10
We next examined the efficacy of IL-10 gene therapy in reversing paclitaxel-induced allodynia. We have recently shown that prolonged reversal of CCI-induced mechanical allodynia could be obtained by two successive i.t. injections of pDNA-IL-10 separated by 3 days (Milligan et al., 2006). The duration of reversal by pDNA-IL-10 is dose-dependent, with doses of 100 and 25 μg given on the first and second injection, respectively, being the most effective in reversing CCI-induced allodynia, lasting for at least 3 months (Sloane et al., in preparation). This dose combination was used in all subsequent experiments. In order to assess potential effects of i.t. injections of plasmid DNA lacking the IL-10 gene, a control plasmid (pDNA-Control) was also tested in some experiments using the same doses.
Baseline responses to the von Frey test showed no differences between groups (Fig. 4). Repeated i.p. injections of paclitaxel (cumulative dose 4 mg/kg) induced reliable bilateral allodynia by 3 weeks after the first injection. Following the paired i.t. injections of pDNA-IL-10 on Days 35 and 38, paclitaxel-treated rats showed a partial, but reliable reversal of allodynia, which lasted through Day 33 after the first pDNA-IL-10 injection (p<0.05 vs. PBS-injected rats for Days 4-33). From Day 37 after the first pDNA-IL-10 injection, allodynia was no longer significantly different between groups.
Fig. 4.
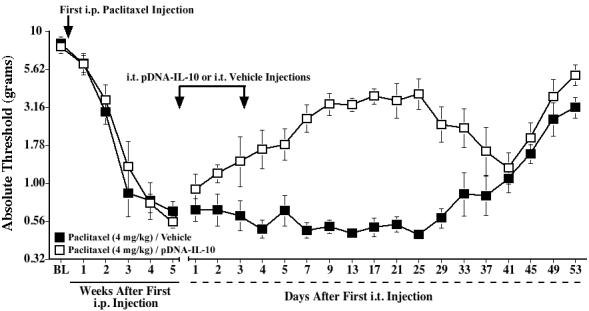
Intrathecal IL-10 gene therapy partially reverses paclitaxel-induced mechanical allodynia. Rats received i.p. injections of paclitaxel (cumulative dose 4 mg/kg) on alternate days, and two i.t. injections of pDNA-IL-10 (100 and 25 μg, respectively) or vehicle were given 5 weeks after the first paclitaxel injection, 3 days apart (indicated by the arrows). Low-threshold mechanical sensitivity was assessed by the von Frey test, before (baseline, BL), weekly after the first paclitaxel injection, and up to 53 days after the first i.t. injection. Data represent means ± SE.
Experiment 5: Prevention of paclitaxel-induced mechanical allodynia by i.t. pDNA-IL-10
In addition, we investigated whether IL-10 gene therapy could also prevent or delay the onset of paclitaxel-induced allodynia. Considering a significant percentage of patients will develop neuropathic pain upon repeated dosing of paclitaxel during the course of treatment (Postma et al., 1995), we explored whether pDNA-IL10 could arrest the further development of allodynia once allodynia was initially observed. In this experiment, IL-10 gene therapy was initiated on Day 12 after the first paclitaxel injection, when rats were just beginning to exhibit mild allodynia. As shown in Fig. 5, PBS-injected controls progress to a more severe allodynia, whereas thresholds in pDNA-IL-10-treated rats remain stable (p<0.05 vs. PBS for Days 15, 17, 24, 27, 30). Thresholds of pDNA-Control-injected rats were not significantly different from either PBS- or pDNA-IL-10-injected groups up to Day 21, but differed from pDNA-IL-10-treated rats thereafter (p<0.05 vs. pDNA-IL-10 for Days 24, 27).
Fig. 5.
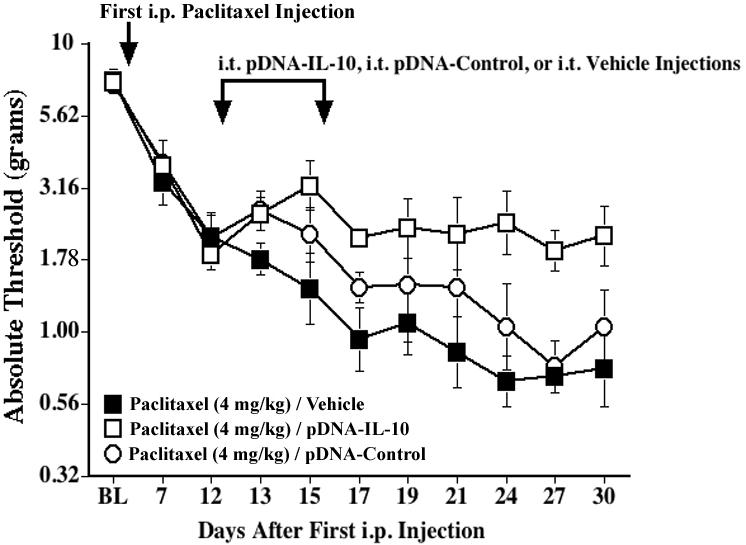
Intrathecal IL-10 gene therapy can arrest the development of mechanical allodynia induced by paclitaxel. Rats received i.p. injections of paclitaxel (cumulative dose 4 mg/kg) on alternate days, and two i.t. injections of pDNA-IL-10 (100 and 25 μg, respectively) or vehicle were given on Days 12 and 15 after the first paclitaxel injection (indicated by the arrows). Low-threshold mechanical sensitivity was assessed by the von Frey test, before (baseline, BL), and up to 30 days after the first paclitaxel injection. Data represent means ± SE.
Experiment 6: Reversal of higher dose paclitaxel-induced mechanical allodynia by i.t. pDNA-IL-10
We further explored whether pDNA-IL-10 was able to reverse allodynia induced by the higher dose of paclitaxel, i.e., 8 mg/kg, to allow for a more prolonged timecourse to be examined. Baseline responses to the von Frey test did not differ between groups (Fig. 6). Repeated i.p. injections of paclitaxel induced reliable bilateral allodynia by 3 weeks after the first injection, as compared to vehicle-injected controls. Following the paired i.t. injections of pDNA-IL-10 on Days 35 and 38, paclitaxel-treated rats again showed a partial reversal of allodynia, which lasted through Day 37 after the first pDNA-IL-10 injection (p<0.05 vs. paclitaxel/PBS for Days 1-37). The response thresholds of animals injected with i.p. vehicle were not affected by i.t. pDNA-IL-10, suggesting that pDNA-IL-10 does not alter normal pain responsivity.
Fig. 6.
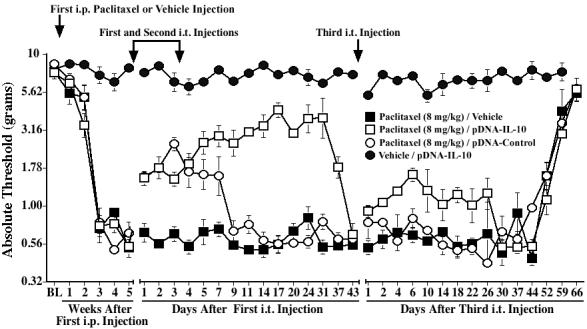
Intrathecal IL-10 gene therapy partially reverses mechanical allodynia induced by a higher dose of paclitaxel. Rats received i.p. injections of paclitaxel (cumulative dose 8 mg/kg) or vehicle on alternate days, and two i.t. injections of pDNA-IL-10 (100 and 25 μg), pDNA-Control (100 and 25 μg), or vehicle were given 5 weeks after the first paclitaxel injection, 3 days apart (indicated by the arrows). Low-threshold mechanical sensitivity was assessed by the von Frey test, before (baseline, BL) and weekly after the first paclitaxel or vehicle injection, and up to 43 days after the first i.t. injection. A third i.t. injection of pDNA-IL-10 (25 μg), pDNA-Control (25 μg), or vehicle was given on Day 43 after the first i.t injection (indicated by the arrow), and von Frey responses were subsequently assessed up to 66 days later. Data represent means ± SE.
The first i.t. injection of pDNA-Control in paclitaxel-treated rats induced a significant reversal of allodynia, which lasted for about 7 days (p<0.05 vs. paclitaxel/PBS for Days 1-7; Fig. 6). Rats returned to allodynia by Day 9 and remained allodynic for the duration of the timecourse. Thus, non-IL-10-encoding plasmid DNA injections lead to a significant though relatively short-lived reversal of allodynia. This observation is consistent with our prior investigations in the CCI model showing that plasmid DNA induces a short-term behavioral reversal of CCI-induced allodynia via spinal release of endogenous IL-10 (Sloane et al., in preparation).
The longer duration of allodynia induced by the higher paclitaxel dose allowed us to examine the efficacy of a third pDNA-IL-10 injection, given after the paclitaxel/pDNA-IL-10-treated group returned to allodynia. This was based on our previous work in the CCI model showing that an additional single pDNA-IL-10 injection is more efficacious in reversing allodynia than it would be if no prior pDNA-IL-10 injections had occurred (Sloane et al., in preparation). Following this third i.t. injection of pDNA-IL-10, a moderate reversal was observed, lasting for approximately 3-4 weeks (p<0.05 vs. paclitaxel/PBS for Days 1-22; Fig. 6).
Experiment 7: Effect of paclitaxel and i.t. pDNA-IL-10 on mRNA expression in lumbar spinal cord, meninges, and DRG
Finally, to examine the mechanism of action of pDNA-IL-10, rats were sacrificed at the time of maximal behavioral reversal of allodynia by i.t. pDNA-IL-10 (18 days after initiation of gene therapy; ∼5 weeks after the first paclitaxel injection) in a separate experiment. Fig. 7A and B show the von Frey assessments, confirming that paclitaxel (cumulative dose 4 mg/kg) leads to reliable mechanical allodynia, as compared to vehicle-injected groups, and that i.t. pDNA-IL-10 and pDNA-Control lead to a prolonged and short-term reversal of allodynia, respectively. As in Experiment 6, neither pDNA-IL-10 nor pDNA-Control significantly affected thresholds of vehicle-injected rats. We assessed mRNA levels of various immune/glial cell markers and cytokines in left lumbar (L4-6) DRG, lumbar meninges, and left spinal cord (Fig. 7C-J). Lumbar DRG were analyzed because paclitaxel can easily access the DRG, who lack a blood-nerve barrier (Devor, 1999), and it has been reported to accumulate there (Cavaletti et al., 2000). Lumbar meninges were collected because data obtained in the CCI model suggest that i.t. delivered plasmid DNA is predominantly taken up by meningeal cells surrounding the lumbosacral CSF space (Milligan et al., 2006).
Fig. 7.
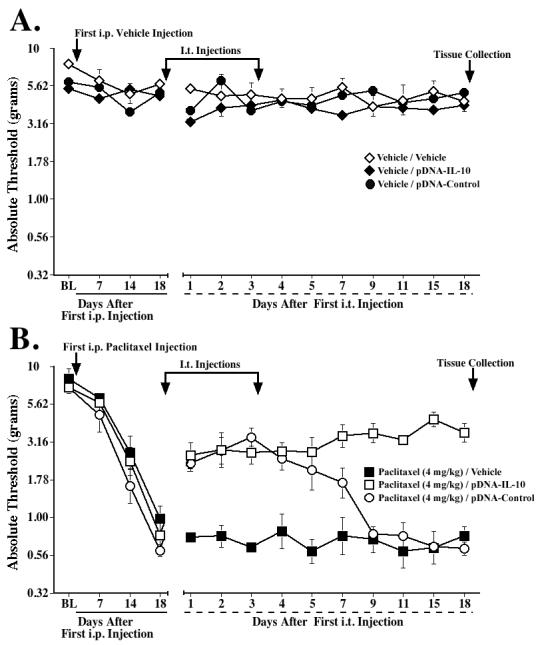
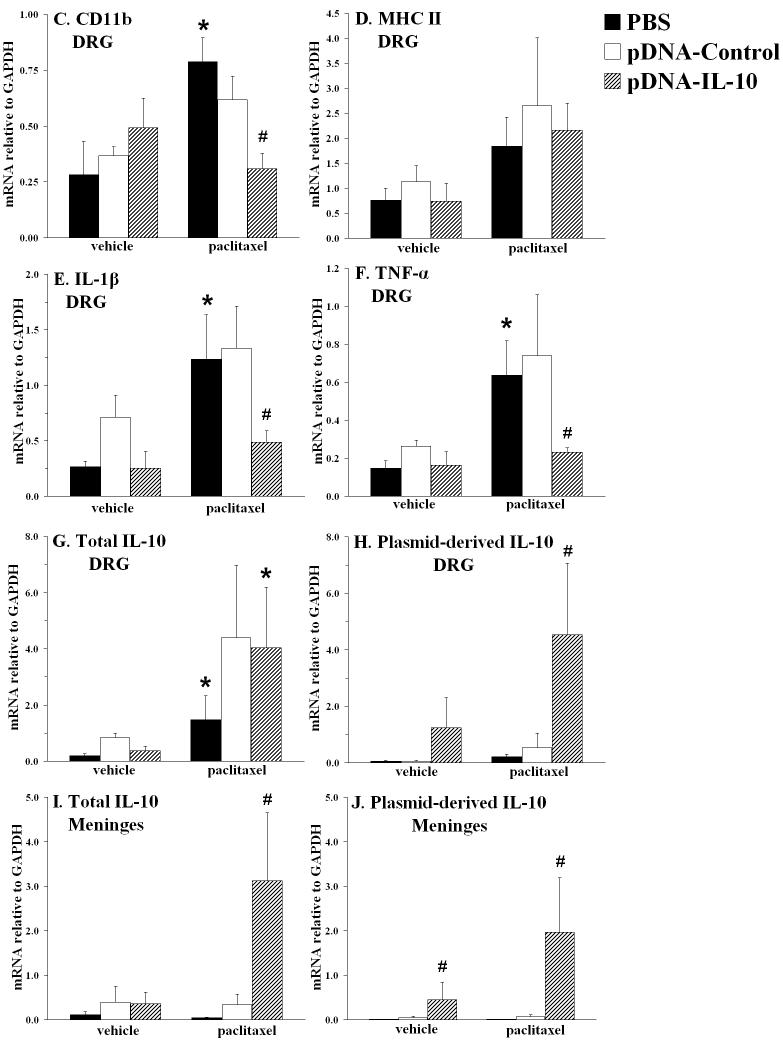
Effects of paclitaxel and IL-10 gene therapy on mRNA expression of immune/glial cell markers and proinflammatory cytokines in lumbar DRG and lumbar meninges. Rats received i.p. vehicle or paclitaxel (cumulative dose 4 mg/kg), and i.t. pDNA-IL-10, pDNA-Control, or vehicle (PBS) on Days 18 and 21 after the first i.p. injection. Assessments on the von Frey test confirmed that these treatments did not affect basal thresholds (A) and that paclitaxel induced mechanical allodynia (B), which was reversed by pDNA-IL-10 (long-term) and pDNA-Control (short-term). Tissues were collected on Day 36 for RT-PCR analysis; C-H show mRNA expression in lumbar DRG of CD11b, MHC class II, IL-1β, TNF-α, total IL-10 and plasmid-derived IL-10, respectively. I and J show mRNA levels in lumbar meninges of total IL-10 and plasmid-derived IL-10, respectively. * p<0.05 vs. respective vehicle group; # p<0.05 vs. paclitaxel/pDNA-Control and vs. paclitaxel/PBS groups. Data represent means ± SE.
Lumbar DRG
In lumbar DRG, mRNA expression was assessed for GFAP, a marker for satellite glial cells, and for CD11b and MHC class II, markers for macrophages and/or dendritic cells. GFAP mRNA levels were similar in all groups (data not shown), whereas CD11b was significantly induced by paclitaxel (Fig. 7C), and MHC II tended to be increased by paclitaxel (Fig. 7D). IL-10 gene therapy did not affect MHC II mRNA, but, in contrast, significantly reduced CD11b mRNA expression levels (p<0.05 vs. paclitaxel/pDNA-Control and vs. paclitaxel/PBS). As shown in Fig. 7E and F, paclitaxel also strongly induced IL-1β and TNF-α mRNA expression, and these increases were inhibited by IL-10 gene therapy (p<0.05 vs. paclitaxel/pDNA-Control and vs. paclitaxel/PBS). Paclitaxel also induced total IL-10 mRNA (Fig. 7G). Gene therapy with either plasmid tended to further increase total IL-10 mRNA. In contrast, using primers specific for plasmid-derived IL-10 mRNA (i.e. produced by the cells that have taken up the plasmid), plasmid-derived IL-10 mRNA was only increased in the paclitaxel/pDNA-IL-10 group (p<0.05 vs. paclitaxel/PBS; Fig. 7H). Notably, almost all of the IL-10 mRNA produced in DRG of paclitaxel/pDNA-IL-10-treated rats appears to be plasmid-derived, as these levels were similar to total IL-10 mRNA levels.
Meninges
In lumbar meninges, CD11b and MHC II mRNA levels were also assessed, as macrophages and dendritic cells are the predominant immune cells found in the meninges. Neither paclitaxel nor IL-10 gene therapy affected CD11b or MHC II expression in this tissue (data not shown). Also, no effect of either paclitaxel or IL-10 gene therapy was observed on IL-1β or TNF-α mRNA expression (data not shown). However, both total IL-10 and plasmid-derived IL-10 were significantly increased in the paclitaxel/pDNA-IL-10-treated group (p<0.05 vs. paclitaxel/pDNA-Control and vs. paclitaxel/PBS; Fig. 7I and J). Plasmid-derived IL-10 mRNA was also induced in rats treated with the vehicle of paclitaxel plus pDNA-IL-10 (p<0.05 vs. vehicle/PBS).
Spinal cord
Since we observed microglial activation in the lumbar spinal cord following paclitaxel as visualized by immunohistochemistry (Exp. 2), CD11b and MHC II mRNA were also analyzed in the spinal cord. No significant paclitaxel-induced changes were found in the levels of either mRNA species. However, CD11b mRNA expression tended to be increased when comparing plasmid-treated groups (both pDNA-Control and pDNA-IL-10) with PBS-treated groups (data not shown). In addition, neither paclitaxel nor IL-10 gene therapy affected mRNA expression of proinflammatory cytokines, total IL-10, or plasmid-derived IL-10 in spinal cord (data not shown).
Discussion
These studies evaluate the efficacy of IL-10 gene therapy in attenuating painful neuropathy in rats produced by the chemotherapeutic paclitaxel. The data demonstrate that intrathecal pDNA-IL-10 reduced paclitaxel-induced bilateral mechanical allodynia (von Frey test) for several weeks. In contrast, control plasmid was only transiently effective. pDNA-IL-10 administered shortly after the last paclitaxel administration delayed subsequent onset and progression of allodynia. This study also provides evidence that the behavioral effects of intrathecal pDNA-IL-10 were associated with decreased expression of the immune cell marker CD11b and the proinflammatory cytokines TNF-α and IL-1β in lumbar DRG.
Clinically, paclitaxel therapy causes a dose-related, predominantly sensory neuropathy, which often progressively worsens after multiple treatments and persists for months (Dougherty et al., 2004; Postma et al., 1995; Quasthoff and Hartung, 2002; Rowinsky et al., 1993). In rodents, relatively high dose paclitaxel regimens cause axonal degeneration, demyelination, and sensory loss (Authier et al., 2000; Cavaletti et al., 1997; Cavaletti et al., 1995; Cliffer et al., 1998; Wang et al., 2002), but lower doses (as used in the present study) induce pain behaviors without marked neuropathology (Flatters and Bennett, 2004; Polomano et al., 2001; Smith et al., 2004). Paclitaxel-induced allodynia/hyperalgesia in rodents can be attenuated by calcium-reducing drugs (Siau and Bennett, 2006), knockdown of the transient receptor potential vanilloid 4 (TRPV4) (Alessandri-Haber et al., 2004) or β1 integrin gene expression in sensory nerves (Dina et al., 2004), and a cannabinoid (Pascual et al., 2005). Glutamate transporter downregulation in the spinal dorsal horn has also been implicated (Weng et al., 2005). Recently, abnormalities in axonal mitochondria of sensory nerves have been suggested to contribute to paclitaxel-induced pain (Flatters and Bennett, 2006).
Here, we confirmed that low paclitaxel doses induce long-lasting tactile allodynia in rats of which the duration, but not magnitude, is dose-dependent (Polomano et al., 2001). We demonstrate that IL-1ra transiently alleviated paclitaxel-induced allodynia with similar duration of reversal (2-3 hours; consistent with its half-life) as previously observed in CCI (Milligan et al., 2005a), suggesting that spinal IL-1/IL-1 receptors are involved in paclitaxel-induced allodynia.
Previously, we observed that one intrathecal pDNA-IL-10 injection reverses CCI-induced allodynia for ∼3-5 days, whereas two injections spaced ∼3 days apart results in long-term (>2 months) behavioral reversal in this model (Milligan et al., 2006). pDNA-IL-10 treatment increased IL-10 mRNA and/or protein in meninges and lumbosacral CSF, compared to controls. Moreover, intrathecal injection of an anti-IL-10 antibody (but not control antibody) blocks the effects of IL-10 gene therapy (Sloane et al., in preparation) suggesting behavioral efficacy is dependent on IL-10. A similar phenomenon occurs in paclitaxel-treated rats, i.e., one intrathecal pDNA-IL-10 injection results in a ∼5-day reversal only (unpublished observations), whereas two injections lead to a partial, but prolonged reversal of paclitaxel-induced allodynia. Possibly, the cellular uptake of pDNA of the second injection may be facilitated by the previous pDNA injection, somehow leading to a ‘primed’ or sensitized state. This may also explain our observation that a third intrathecal pDNA-IL-10 injection produces a more prolonged reversal than a single injection would if no prior pDNA-IL-10 injections had occurred.
Animals treated with pDNA-Control showed a short-term behavioral reversal. This agrees with our prior observations in the CCI model (Sloane et al., in preparation), that also indicate that this effect was dependent on the induction of endogenous IL-10, as co-treatment with anti-IL-10 antibody blocked it. That study also showed that an intrathecal pDNA-Control injection induced IL-10 protein levels in CSF compared to control rats, 3 days post-injection. This anti-inflammatory response may be due to bacterial-derived unmethylated cytosine-linked guanine dinucleotide (CpG) motifs in the pDNA, which activate macrophages and dendritic cells via Toll-like receptor 9 (TLR9) to initiate innate immune responses (Gordon, 2002; Krieg, 2002). Through an alternative activation pathway, this response may suppress inflammation (Goerdt and Orfanos, 1999; Gordon, 2003).
Our investigation of the mechanism of action of IL-10 gene therapy revealed that pDNA-IL-10 decreased paclitaxel-induced mRNA expression of CD11b, a macrophage/dendritic cell marker, in lumbar DRG. Both types of immune cells are found in healthy DRG (Lu and Richardson, 1993; Olsson, 1990). However, peripheral nerve injury induces further infiltration of these cells into the DRG (Hu and McLachlan, 2002; Lu and Richardson, 1993). Thus, our data suggest that paclitaxel either activates resident CD11b+ immune cells, or increases their recruitment.
The data further show that paclitaxel treatment markedly induces TNF-α and IL-1β mRNA levels in lumbar DRG, which were decreased in rats treated with pDNA-IL-10, but not those treated with pDNA-Control. Although TNF-α and IL-1β can be expressed by DRG neurons in both naïve and neuropathic rats (Copray et al., 2001; Li et al., 2004; Schafers et al., 2003a), we speculate that they are predominantly derived from macrophages, as their decreased expression in pDNA-IL-10-treated rats correlates with decreased CD11b expression within the same rats. Regardless of the cellular source(s) of TNF and IL-1, both can act directly on DRG neurons in vitro (Inoue et al., 1999; Pollock et al., 2002), and receptors for these cytokines are identified on DRG neuronal cell bodies (Copray et al., 2001; Li et al., 2004; Pollock et al., 2002). Also, TNF injected into lumbar DRG of naïve rats elicits long-lasting allodynia, and further enhances allodynia induced by DRG compression or spinal nerve injury (Homma et al., 2002; Schafers et al., 2003b). Proinflammatory cytokines may thus sensitize primary sensory afferents, thereby modifying sensory input to the spinal dorsal horn to facilitate pain. Together, our findings suggest that in pDNA-IL-10-treated rats either resident CD11b-positive macrophages are less activated, and therefore express lower levels of proinflammatory cytokines, and/or the number of recruited macrophages is decreased.
Our results further indicate that in lumbar meninges total and plasmid-derived IL-10 mRNA were markedly induced in pDNA-IL-10-treated rats compared to either vehicle- or pDNA-Control-treated animals, but not in lumbar cord. This is consistent with data from CCI animals, showing that, upon i.t. injection, pDNA is predominantly expressed by meningeal cells, presumably macrophages and dendritic cells, surrounding the injection site, but not detected in spinal cord parenchyma except for superficial layers of the dorsal horn (Milligan et al., 2006). Interestingly, we also detected plasmid-derived IL-10 mRNA in lumbar DRG. At present it is unclear whether pDNA-IL-10 reaches the DRG via retrograde transport from the spinal cord, is taken up directly by DRG cells from the CSF, or is taken up by meningeal cells very closely associated with the DRG.
Paclitaxel did not affect immune or glial cell marker or cytokine expression in meninges and spinal cord, possibly because any changes in these tissues were too subtle or too localized to be detected at this time point. However, since IL-10 receptors are expressed on microglial cells (Ledeboer et al., 2002; Mizuno et al., 1994), and suggested by our immunohistochemical evaluation, we speculate that spinal cord activated microglia may be involved. Recent reports show that i.p. propentofylline, thalidomide, and minocycline, all glial activation inhibitors that cross the blood-brain barrier, of which minocycline is considered to be selective for microglia, attenuate paclitaxel- or vincristine-induced neuropathic pain (Cata et al., 2006; Sweitzer et al., 2006), supporting a role for activated (micro)glial cells in these conditions. However, since these compounds were systemically administered, effects on peripheral nerves and/or DRG can not be excluded.
To what extent paclitaxel and/or pDNA-IL-10 in our current model target cells in the DRG, CSF, and/or spinal cord, remains to be investigated. Overall, our data are consistent with recent research showing intravenous paclitaxel induces the number of activated macrophages within the DRG and microglial activation in the lumbar spinal cord (Peters et al., 2006). Moreover, in vitro studies indicate that paclitaxel activates macrophages in a lipopolysaccharide (LPS)-like manner, f.e., it induces TNF-α and IL-1β expression (Bogdan and Ding, 1992; Ding et al., 1990; Moos and Fitzpatrick, 1998). These actions can be inhibited by IL-10 (Bogdan and Ding, 1992; Mullins et al., 2001) and appear to involve the LPS recognition/signaling receptors CD14, CD11b, and Toll-like receptor 4 (TLR4) (Byrd-Leifer et al., 2001; Perera et al., 2001). Microglia also express these receptors, and particularly TLR-4 has been implicated as a key receptor in the initiation of nerve injury-induced behavioral hypersensitivity (Tanga et al., 2005).
In conclusion, we demonstrate that paclitaxel-induced painful neuropathy can be attenuated by intrathecal IL-10 gene therapy. Our data strongly suggest pDNA-IL-10 acts at least at the level of the DRG, as its prolonged suppression of paclitaxel-induced allodynia is associated with decreased CD11b, TNF-α, and IL-1β expression in lumbar DRG, suggesting IL-10 inhibits activation and/or recruitment of CD11b-positive immune cells into the DRG and subsequent proinflammatory cytokine production. This non-viral gene therapy may represent a novel approach for the control of paclitaxel-induced neuropathic pain. Since the clinical presentation and time course of neuropathic pain is remarkably similar for all classes of chemotherapeutic drugs, and chemotherapy-induced sensory disturbances may result from shared/convergent mechanisms (Cata et al., 2006), we speculate that IL-10 gene therapy may also be efficacious in attenuating painful neuropathies induced by other chemotherapeutics.
Acknowledgements
This work was supported by Avigen and NIH grants DA018156 and DA015642. We thank Amgen (Thousand Oaks, CA, USA) for the gift of IL-1ra and vehicle.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
A.L., K.W.J., and R.A.C. are currently employees of and have stock options in Avigen, Inc.
D.M. is currently an employee of Amgen. L.R.W. has received research support by Avigen, Inc.
References
- Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neurosci. 2004;24:4444–4452. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. Description of a short-term Taxol-induced nociceptive neuropathy in rats. Brain Res. 2000;887:239–249. doi: 10.1016/s0006-8993(00)02910-3. [DOI] [PubMed] [Google Scholar]
- Bogdan C, Ding A. Taxol, a microtubule-stabilizing antineoplastic agent, induces expression of tumor necrosis factor alpha and interleukin-1 in macrophages. J Leukoc Biol. 1992;52:119–121. doi: 10.1002/jlb.52.1.119. [DOI] [PubMed] [Google Scholar]
- Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur J Immunol. 2001;31:2448–2457. doi: 10.1002/1521-4141(200108)31:8<2448::aid-immu2448>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Cata JP, Weng HR, Lee BN, Reuben JM, Dougherty PM. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiol. 2006;72:151–169. [PubMed] [Google Scholar]
- Cavaletti G, Cavalletti E, Montaguti P, Oggioni N, De Negri O, Tredici G. Effect on the peripheral nervous system of the short-term intravenous administration of paclitaxel in the rat. Neurotoxicology. 1997;18:137–145. [PubMed] [Google Scholar]
- Cavaletti G, Cavalletti E, Oggioni N, Sottani C, Minoia C, D’Incalci M, Zucchetti M, Marmiroli P, Tredici G. Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicology. 2000;21:389–393. [PubMed] [Google Scholar]
- Cavaletti G, Tredici G, Braga M, Tazzari S. Experimental peripheral neuropathy induced in adult rats by repeated intraperitoneal administration of taxol. Exp Neurol. 1995;133:64–72. doi: 10.1006/exnr.1995.1008. [DOI] [PubMed] [Google Scholar]
- Chacur M, Milligan ED, Sloan EM, Wieseler-Frank J, Barrientos RM, Martin D, Poole S, Lomonte B, Gutierrez JM, Maier SF, Cury Y, Watkins LR. Snake venom phospholipase A2s (Asp49 and Lys49) induce mechanical allodynia upon peri-sciatic administration: involvement of spinal cord glia, proinflammatory cytokines and nitric oxide. Pain. 2004;108:180–191. doi: 10.1016/j.pain.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Siuciak JA, Carson SR, Radley HE, Park JS, Lewis DR, Zlotchenko E, Nguyen T, Garcia K, Tonra JR, Stambler N, Cedarbaum JM, Bodine SC, Lindsay RM, DiStefano PS. Physiological characterization of Taxol-induced large-fiber sensory neuropathy in the rat. Ann Neurol. 1998;43:46–55. doi: 10.1002/ana.410430111. [DOI] [PubMed] [Google Scholar]
- Copray JC, Mantingh I, Brouwer N, Biber K, Kust BM, Liem RS, Huitinga I, Tilders FJ, Van Dam AM, Boddeke HW. Expression of interleukin-1 beta in rat dorsal root ganglia. J Neuroimmunol. 2001;118:203–211. doi: 10.1016/s0165-5728(01)00324-1. [DOI] [PubMed] [Google Scholar]
- Devor M. Unexplained peculiarities of the dorsal root ganglion. Pain Suppl. 1999;6:S27–35. doi: 10.1016/S0304-3959(99)00135-9. [DOI] [PubMed] [Google Scholar]
- Dina OA, Parada CA, Yeh J, Chen X, McCarter GC, Levine JD. Integrin signaling in inflammatory and neuropathic pain in the rat. Eur J Neurosci. 2004;19:634–642. doi: 10.1111/j.1460-9568.2004.03169.x. [DOI] [PubMed] [Google Scholar]
- Ding AH, Porteu F, Sanchez E, Nathan CF. Shared actions of endotoxin and taxol on TNF receptors and TNF release. Science. 1990;248:370–372. doi: 10.1126/science.1970196. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Flatters SJ, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109:150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Flatters SJ, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006;122:245–257. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Harvey LO. Efficient estimation of sensory thresholds. Behav. Res. Meth. Instrum. Comput. 1986;18:623–632. [Google Scholar]
- Homma Y, Brull SJ, Zhang JM. A comparison of chronic pain behavior following local application of tumor necrosis factor alpha to the normal and mechanically compressed lumbar ganglia in the rat. Pain. 2002;95:239–246. doi: 10.1016/S0304-3959(01)00404-3. [DOI] [PubMed] [Google Scholar]
- Hu P, McLachlan EM. Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience. 2002;112:23–38. doi: 10.1016/s0306-4522(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Inoue A, Ikoma K, Morioka N, Kumagai K, Hashimoto T, Hide I, Nakata Y. Interleukin-1beta induces substance P release from primary afferent neurons through the cyclooxygenase-2 system. J Neurochem. 1999;73:2206–2213. [PubMed] [Google Scholar]
- Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- Laughlin TM, Bethea JR, Yezierski RP, Wilcox GL. Cytokine involvement in dynorphin-induced allodynia. Pain. 2000;84:159–167. doi: 10.1016/s0304-3959(99)00195-5. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Breve JJ, Wierinckx A, van der Jagt S, Bristow AF, Leysen JE, Tilders FJ, Van Dam AM. Expression and regulation of interleukin-10 and interleukin-10 receptor in rat astroglial and microglial cells. Eur J Neurosci. 2002;16:1175–1185. doi: 10.1046/j.1460-9568.2002.02200.x. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Li Y, Ji A, Weihe E, Schafer MK. Cell-specific expression and lipopolysaccharide-induced regulation of tumor necrosis factor alpha (TNFalpha) and TNF receptors in rat dorsal root ganglion. J Neurosci. 2004;24:9623–9631. doi: 10.1523/JNEUROSCI.2392-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu X, Richardson PM. Responses of macrophages in rat dorsal root ganglia following peripheral nerve injury. J Neurocytol. 1993;22:334–341. doi: 10.1007/BF01195557. [DOI] [PubMed] [Google Scholar]
- Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Langer SJ, Sloane EM, He L, Wieseler-Frank J, O’Connor K, Martin D, Forsayeth JR, Maier SF, Johnson K, Chavez RA, Leinwand LA, Watkins LR. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci. 2005a;21:2136–2148. doi: 10.1111/j.1460-9568.2005.04057.x. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Sloane EM, Langer SJ, Cruz PE, Chacur M, Spataro L, Wieseler-Frank J, Hammack SE, Maier SF, Flotte TR, Forsayeth JR, Leinwand LA, Chavez R, Watkins LR. Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Mol Pain. 2005b;1:9. doi: 10.1186/1744-8069-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Sloane EM, Langer SJ, Hughes TS, Jekich BM, Frank MG, Mahoney JH, Levkoff LH, Maier SF, Cruz PE, Flotte TR, Johnson KW, Mahoney MM, Chavez RA, Leinwand LA, Watkins LR. Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain. 2006 doi: 10.1016/j.pain.2006.07.009. in press.
- Milligan ED, Twining C, Chacur M, Biedenkapp J, O’Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Sawada M, Marunouchi T, Suzumura A. Production of interleukin-10 by mouse glial cells in culture. Biochem Biophys Res Commun. 1994;205:1907–1915. doi: 10.1006/bbrc.1994.2893. [DOI] [PubMed] [Google Scholar]
- Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Brain Res Rev. 2006;51:240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Moos PJ, Fitzpatrick FA. Taxane-mediated gene induction is independent of microtubule stabilization: induction of transcription regulators and enzymes that modulate inflammation and apoptosis. Proc Natl Acad Sci U S A. 1998;95:3896–3901. doi: 10.1073/pnas.95.7.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins DW, Martins RS, Burger CJ, Elgert KD. Tumor cell-derived TGF-beta and IL-10 dysregulate paclitaxel-induced macrophage activation. J Leukoc Biol. 2001;69:129–137. [PubMed] [Google Scholar]
- Olsson Y. Microenvironment of the peripheral nervous system under normal and pathological conditions. Crit Rev Neurobiol. 1990;5:265–311. [PubMed] [Google Scholar]
- Pascual D, Goicoechea C, Suardiaz M, Martin MI. A cannabinoid agonist, WIN 55,212-2, reduces neuropathic nociception induced by paclitaxel in rats. Pain. 2005;118:23–34. doi: 10.1016/j.pain.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Perera PY, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, Goyert SM, Vogel SN. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J Immunol. 2001;166:574–581. doi: 10.4049/jimmunol.166.1.574. [DOI] [PubMed] [Google Scholar]
- Peters CM, Jimenez-Andrade JM, Jonas BM, Sevcik MA, Koewler NJ, Ghilardi JR, Wong GY, Mantyh PW. Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp Neurol. 2006 doi: 10.1016/j.expneurol.2006.07.022. in press.
- Pollock J, McFarlane SM, Connell MC, Zehavi U, Vandenabeele P, MacEwan DJ, Scott RH. TNF-alpha receptors simultaneously activate Ca2+ mobilisation and stress kinases in cultured sensory neurones. Neuropharmacology. 2002;42:93–106. doi: 10.1016/s0028-3908(01)00163-0. [DOI] [PubMed] [Google Scholar]
- Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6:489–494. doi: 10.1093/oxfordjournals.annonc.a059220. [DOI] [PubMed] [Google Scholar]
- Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol) Semin Oncol. 1993;20:1–15. [PubMed] [Google Scholar]
- Schafers M, Geis C, Svensson CI, Luo ZD, Sommer C. Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur J Neurosci. 2003a;17:791–804. doi: 10.1046/j.1460-9568.2003.02504.x. [DOI] [PubMed] [Google Scholar]
- Schafers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J Neurosci. 2003b;23:3028–3038. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siau C, Bennett GJ. Dysregulation of cellular calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth Analg. 2006;102:1485–1490. doi: 10.1213/01.ane.0000204318.35194.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Crager SE, Mogil JS. Paclitaxel-induced neuropathic hypersensitivity in mice: responses in 10 inbred mouse strains. Life Sci. 2004;74:2593–2604. doi: 10.1016/j.lfs.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, Dantzer R, Kelley KW. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–449. [PubMed] [Google Scholar]
- Sweitzer SM, Pahl JL, Deleo JA. Propentofylline attenuates vincristine-induced peripheral neuropathy in the rat. Neurosci Lett. 2006;400:258–261. doi: 10.1016/j.neulet.2006.02.058. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutwein B, Strasburger H. Fitting the psychometric function. Percept Psychophys. 1999;61:87–106. doi: 10.3758/bf03211951. [DOI] [PubMed] [Google Scholar]
- Wang MS, Davis AA, Culver DG, Glass JD. WldS mice are resistant to paclitaxel (taxol) neuropathy. Ann Neurol. 2002;52:442–447. doi: 10.1002/ana.10300. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Martin D, Ulrich P, Tracey KJ, Maier SF. Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. Pain. 1997;71:225–235. doi: 10.1016/s0304-3959(97)03369-1. [DOI] [PubMed] [Google Scholar]
- Weng HR, Aravindan N, Cata JP, Chen JH, Shaw AD, Dougherty PM. Spinal glial glutamate transporters downregulate in rats with taxol-induced hyperalgesia. Neurosci Lett. 2005;386:18–22. doi: 10.1016/j.neulet.2005.05.049. [DOI] [PubMed] [Google Scholar]